2. Two students are having an argument: Student A: If I combine hydrogen and oxygen, I get water. Student B: No, you don't. If you combine hydrogen and oxygen, you get a mixture of hydrogen and oxygen. Dr. H: Well, you are both right. Whether you get a compound or a mixture depends on how you combine them. Using what you learned about changes in matter, explain how each student can be correct: Student A: Student B:
2. Two students are having an argument: Student A: If I combine hydrogen and oxygen, I get water. Student B: No, you don't. If you combine hydrogen and oxygen, you get a mixture of hydrogen and oxygen. Dr. H: Well, you are both right. Whether you get a compound or a mixture depends on how you combine them. Using what you learned about changes in matter, explain how each student can be correct: Student A: Student B:
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter3: Matter
Section: Chapter Questions
Problem 5ALQ: Sketch a magnified view (showing atoms and/or molecules) of each of the following, and explain why...
Related questions
Question
Please Help, Thank you.
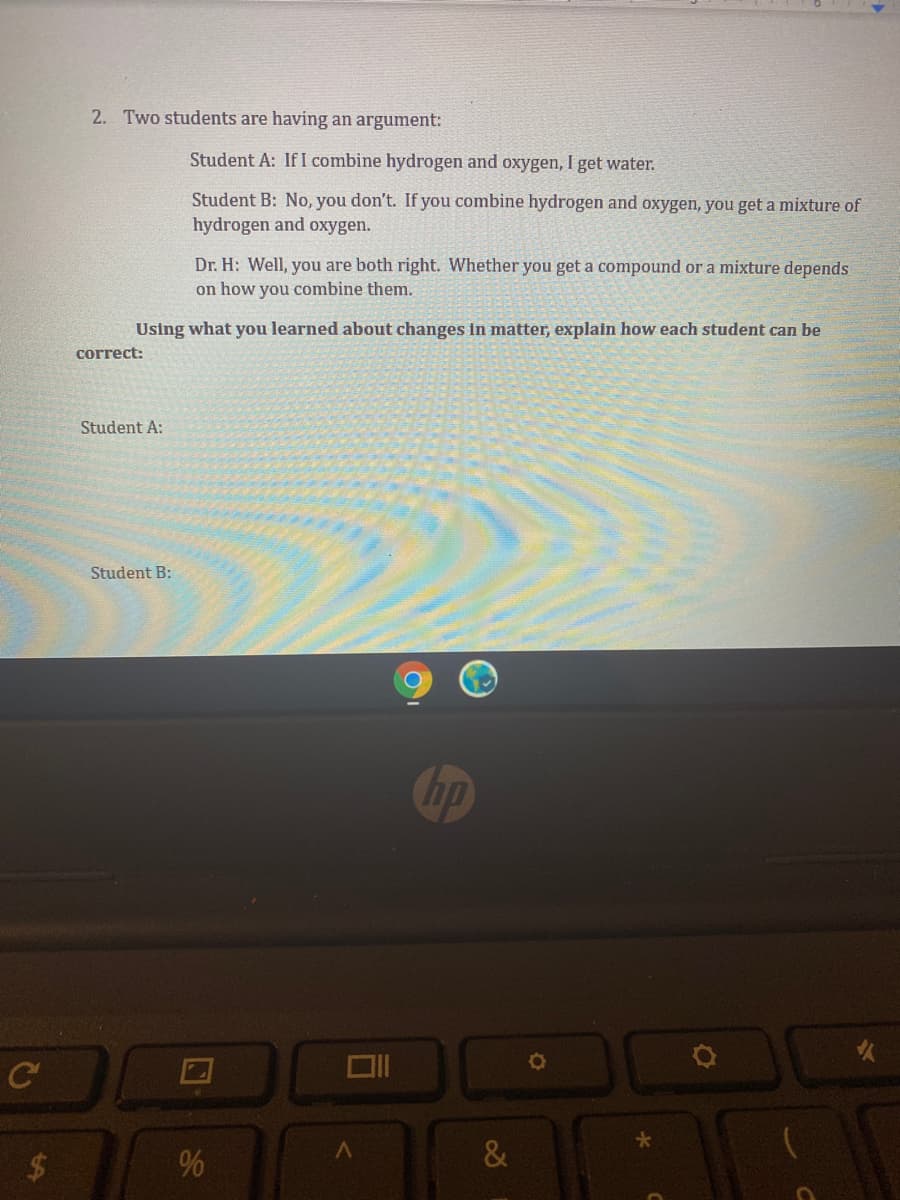
Transcribed Image Text:2. Two students are having an argument:
Student A: If I combine hydrogen and oxygen, I get water.
Student B: No, you don't. If you combine hydrogen and oxygen, you get a mixture of
hydrogen and oxygen.
Dr. H: Well, you are both right. Whether you get a compound or a mixture depends
on how you combine them.
Using what you learned about changes in matter, explain how each student can be
correct:
Student A:
Student B:
Chp
C
&
2$4
Expert Solution
Step 1
In this question, methods for obtaining mixture and water on combination of hydrogen and oxygen is to be determined.
Step 2 Formation of mixture
At room temperature, when hydrogen and oxygen gases are mixed together, they forms only a mixture of gases. This is because there is no reaction occurs between these molecules. For the reaction to occur, it requires an activation energy which is not available at room temperature.
Thus, student A is correct, if hydrogen and oxygen is combined at room temperature only.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
