2. Using data from Table, calculate the daily energy needs of a person who sleeps for 8.00 h, walks for 2.00 h, works in the office for 8 hours, does moderate physical work during 2 hours and lying awake for 4 hours. (suppose, that working in office consumes energy at the same rate as sitting upright.). Suppose that the person mass is 80-kg and his height 1.75m. 1. First law of thermodinamics AQ = AU + AW The first law of thermodynamics is a version of the law of conservation of energy The first law is often formulated by stating that the change in the internal energy AU of a closed system is equal to the amount of heat supplied to the system AQ, minus the amount of work done AW by the system on its surroundings. Units of work and energy are Joules(J) and calories (cal). Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J Kinetic energy (J): KE = , here m is mass of a body (kg), v – speed of its motion(m/sec). 2. 3. Gravitational potential energy(J): PE = mgh, here m is mass of the body (kg); g = 9,8 m/sec? is acceleration due to gravity; h is height (m). Work W (J): 4. Formally, the work done on a system by a constant force is defined to be the product of the component of the force in the direction of motion times the distance through which the force acts. For one-way motion in one dimension, this is expressed in equation form as: W = FAxcos0 , where W is work, Ax is the displacement of the system, and 6 is the angle between the force vector F and the displacement vector Ax. 5. Work – Energy theorem: The principle of work and kinetic energy (also known as the work-energy theorem) states that the work done by the sum of all forces acting on a particle equals the change in the kinetic energy of the particle: W = AKE = "-i, here v, is final velocity and v; is initial velocity. Let us calculate the work done in lifting an object of mass m through a height h. If the object is lifted straight up at constant speed, then the force needed to lift it is equal to its weight mg. The work done on the mass is: W = APE = mgAh, here m is mass of the body (kg); g = 9,8 m/sec? is acceleration due to gravity; Ah is change in height. 6. Power P (W) is rate at which work is done: P = ", where W is work (J) and t is time. 7. Efficiency Even though energy is conserved in an energy conversion process, the output of useful energy or work will be less than the energy input. The efficiency n of an energy conversion process is defined as 1 = - where W is useful energy or work output, E is total energy input, Q is heat or thermal w-Q energy. 8. Energy conversion in humans Our own bodies, like all living organisms, are energy conversion machines. Conservation of energy implies that the chemical energy stored in food is converted into work, thermal energy, and/or stored as chemical energy in fatty tissue. The fraction going into each form depends both on how much we eat and on our level of physical activity. If we eat more than is needed to do work and stay warm, the remainder goes into body fat. carbohydrates and proteins K1 = 17,2 kJ/g =4.11 Cal/g fat K2 = 38,9 kJ/g=9.3 Cal/g. W (negative) Work OE Food Thermal energy energy OE, Stored fat OE, + W = OE,
2. Using data from Table, calculate the daily energy needs of a person who sleeps for 8.00 h, walks for 2.00 h, works in the office for 8 hours, does moderate physical work during 2 hours and lying awake for 4 hours. (suppose, that working in office consumes energy at the same rate as sitting upright.). Suppose that the person mass is 80-kg and his height 1.75m. 1. First law of thermodinamics AQ = AU + AW The first law of thermodynamics is a version of the law of conservation of energy The first law is often formulated by stating that the change in the internal energy AU of a closed system is equal to the amount of heat supplied to the system AQ, minus the amount of work done AW by the system on its surroundings. Units of work and energy are Joules(J) and calories (cal). Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J Kinetic energy (J): KE = , here m is mass of a body (kg), v – speed of its motion(m/sec). 2. 3. Gravitational potential energy(J): PE = mgh, here m is mass of the body (kg); g = 9,8 m/sec? is acceleration due to gravity; h is height (m). Work W (J): 4. Formally, the work done on a system by a constant force is defined to be the product of the component of the force in the direction of motion times the distance through which the force acts. For one-way motion in one dimension, this is expressed in equation form as: W = FAxcos0 , where W is work, Ax is the displacement of the system, and 6 is the angle between the force vector F and the displacement vector Ax. 5. Work – Energy theorem: The principle of work and kinetic energy (also known as the work-energy theorem) states that the work done by the sum of all forces acting on a particle equals the change in the kinetic energy of the particle: W = AKE = "-i, here v, is final velocity and v; is initial velocity. Let us calculate the work done in lifting an object of mass m through a height h. If the object is lifted straight up at constant speed, then the force needed to lift it is equal to its weight mg. The work done on the mass is: W = APE = mgAh, here m is mass of the body (kg); g = 9,8 m/sec? is acceleration due to gravity; Ah is change in height. 6. Power P (W) is rate at which work is done: P = ", where W is work (J) and t is time. 7. Efficiency Even though energy is conserved in an energy conversion process, the output of useful energy or work will be less than the energy input. The efficiency n of an energy conversion process is defined as 1 = - where W is useful energy or work output, E is total energy input, Q is heat or thermal w-Q energy. 8. Energy conversion in humans Our own bodies, like all living organisms, are energy conversion machines. Conservation of energy implies that the chemical energy stored in food is converted into work, thermal energy, and/or stored as chemical energy in fatty tissue. The fraction going into each form depends both on how much we eat and on our level of physical activity. If we eat more than is needed to do work and stay warm, the remainder goes into body fat. carbohydrates and proteins K1 = 17,2 kJ/g =4.11 Cal/g fat K2 = 38,9 kJ/g=9.3 Cal/g. W (negative) Work OE Food Thermal energy energy OE, Stored fat OE, + W = OE,
College Physics
1st Edition
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:Paul Peter Urone, Roger Hinrichs
Chapter7: Work, Energy, And Energy Resources
Section: Chapter Questions
Problem 49PE: Using data from Table 7.5, calculate the daily energy needs of a person who sleeps for 7.00 h, walks...
Related questions
Question
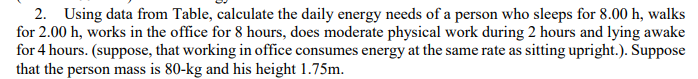
Transcribed Image Text:2. Using data from Table, calculate the daily energy needs of a person who sleeps for 8.00 h, walks
for 2.00 h, works in the office for 8 hours, does moderate physical work during 2 hours and lying awake
for 4 hours. (suppose, that working in office consumes energy at the same rate as sitting upright.). Suppose
that the person mass is 80-kg and his height 1.75m.

Transcribed Image Text:1. First law of thermodinamics AQ = AU + AW
The first law of thermodynamics is a version of the law of conservation of energy The first law is often
formulated by stating that the change in the internal energy AU of a closed system is equal to the amount
of heat supplied to the system AQ, minus the amount of work done AW by the system on its
surroundings.
Units of work and energy are Joules(J) and calories (cal).
Relationship between units 1 cal = 4.18 J and 1 Cal = 1000 cal = 4180 J
Kinetic energy (J): KE = , here m is mass of a body (kg), v – speed of its motion(m/sec).
2.
3. Gravitational potential energy(J): PE = mgh, here m is mass of the body (kg); g = 9,8 m/sec? is
acceleration due to gravity; h is height (m).
Work W (J):
4.
Formally, the work done on a system by a constant force is defined to be the product of the component
of the force in the direction of motion times the distance through which the force acts. For one-way motion
in one dimension, this is expressed in equation form as:
W = FAxcos0 , where W is work, Ax is the displacement of the system, and 6 is the angle between the
force vector F and the displacement vector Ax.
5. Work – Energy theorem: The principle of work and kinetic energy (also known as the work-energy
theorem) states that the work done by the sum of all forces acting on a particle equals the change in the
kinetic energy of the particle: W = AKE = "-i, here v, is final velocity and v; is initial velocity.
Let us calculate the work done in lifting an object of mass m through a height h. If the object is lifted
straight up at constant speed, then the force needed to lift it is equal to its weight mg. The work done on
the mass is: W = APE = mgAh, here m is mass of the body (kg); g = 9,8 m/sec? is acceleration due to
gravity; Ah is change in height.
6. Power P (W) is rate at which work is done: P = ", where W is work (J) and t is time.
7. Efficiency Even though energy is conserved in an energy conversion process, the output of useful
energy or work will be less than the energy input. The efficiency n of an energy conversion process is
defined as 1 =
- where W is useful energy or work output, E is total energy input, Q is heat or thermal
w-Q
energy.
8. Energy conversion in humans
Our own bodies, like all living organisms, are energy conversion
machines. Conservation of energy implies that the chemical
energy stored in food is converted into work, thermal energy,
and/or stored as chemical energy in fatty tissue. The fraction
going into each form depends both on how much we eat and on
our level of physical activity. If we eat more than is needed to
do work and stay warm, the remainder goes into body fat.
carbohydrates and proteins K1 = 17,2 kJ/g =4.11 Cal/g
fat K2 = 38,9 kJ/g=9.3 Cal/g.
W (negative)
Work
OE
Food
Thermal
energy
energy
OE,
Stored
fat
OE, + W = OE,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning