2.1 Consider the table below. Compare the boiling points of 3 molecules with the same molar mass. Mm bp, °C C,H, 44 231 0.1 CH,CHO 44 294 2.7 CH,CN 41 355 3.9 P is the bond dipole which is a measure of polarity of a bond in a molecule
2.1 Consider the table below. Compare the boiling points of 3 molecules with the same molar mass. Mm bp, °C C,H, 44 231 0.1 CH,CHO 44 294 2.7 CH,CN 41 355 3.9 P is the bond dipole which is a measure of polarity of a bond in a molecule
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.45PAE: 8.45 Describe how interactions between molecules affect the vapor pressure of a liquid.
Related questions
Question
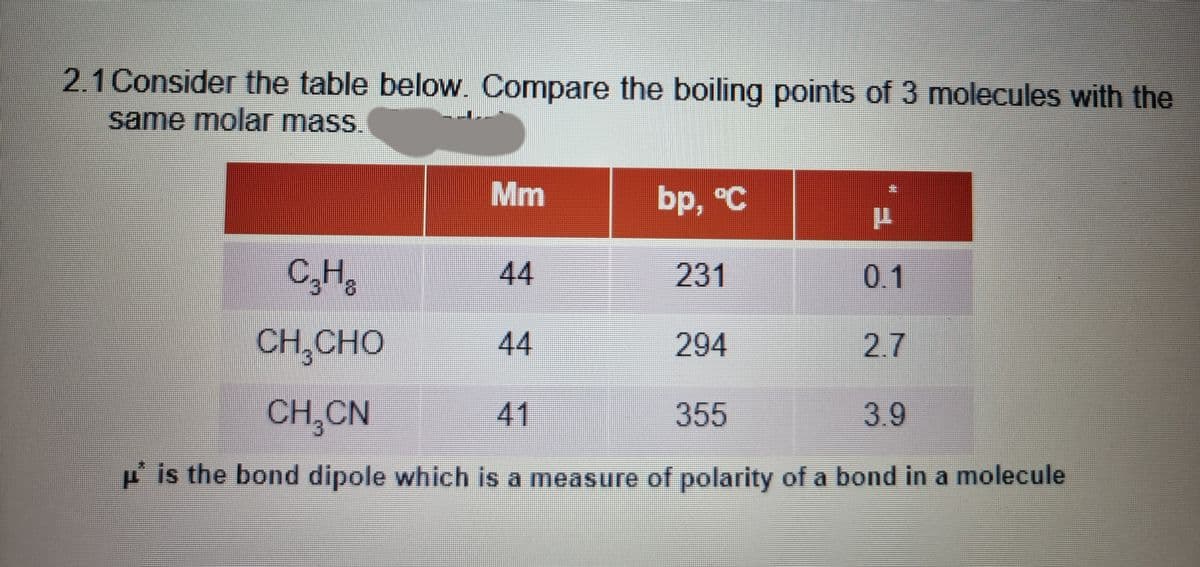
Transcribed Image Text:2.1Consider the table below. Compare the boiling points of 3 molecules with the
same molar mass
Mm
bp, °C
C,H,
44
231
0.1
CH,CHO
44
294
2.7
CH CN
41
355
3.9
P is the bond dipole which is a measure of polarity of a bond in a molecule
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning