2.25 moles of an ideal gas with Cy 3 R under a constant external pressure of 200.0x103 Pa is cooled from 122 to 28.5°C. Calculate q, w, AU, and AH.
2.25 moles of an ideal gas with Cy 3 R under a constant external pressure of 200.0x103 Pa is cooled from 122 to 28.5°C. Calculate q, w, AU, and AH.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.88E: The following are values of heat capacity for nitrogen gas; Temp K Cv J/mol. K 300 20.8 400 20.9 500...
Related questions
Question
100%
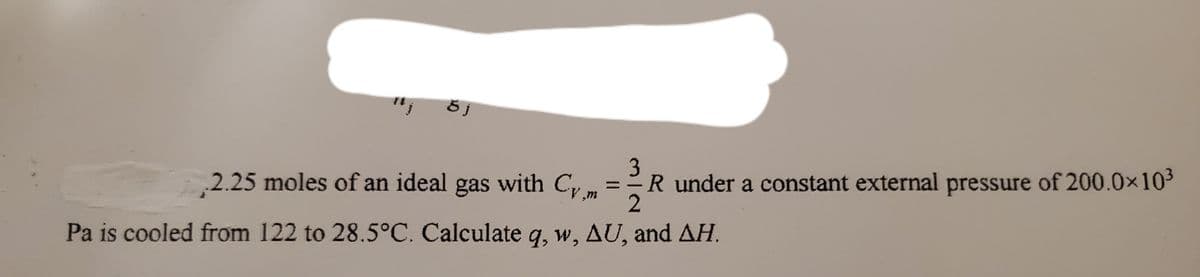
Transcribed Image Text:ŏ j
2.25 moles of an ideal gas with C m
3
R under a constant external pressure of 200.0x103
Pa is cooled from 122 to 28.5°C. Calculate q, w, AU, and AH.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning