20 Earth infrared radiation at the surface Earth infared radiation reaching outer space 15 co, co, CH, HO 10 Wavelength (um) Figure F12-7-3 A. Why is the energy radiated from the earth much lower energy than the light radiated from the sun? B. Why doesn't all of the infrared light escape to outer space? C. The area beneath the curves corresponds to the total amount of energy radiated. The green area is much greater than that of the red. What does this effect mean for the overall AE of Earth? (urluM u) xny angepe
20 Earth infrared radiation at the surface Earth infared radiation reaching outer space 15 co, co, CH, HO 10 Wavelength (um) Figure F12-7-3 A. Why is the energy radiated from the earth much lower energy than the light radiated from the sun? B. Why doesn't all of the infrared light escape to outer space? C. The area beneath the curves corresponds to the total amount of energy radiated. The green area is much greater than that of the red. What does this effect mean for the overall AE of Earth? (urluM u) xny angepe
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 3PS: Traffic signals are often now made of LEDs (light-emitting diodes). Amber and green ones are...
Related questions
Question
100%
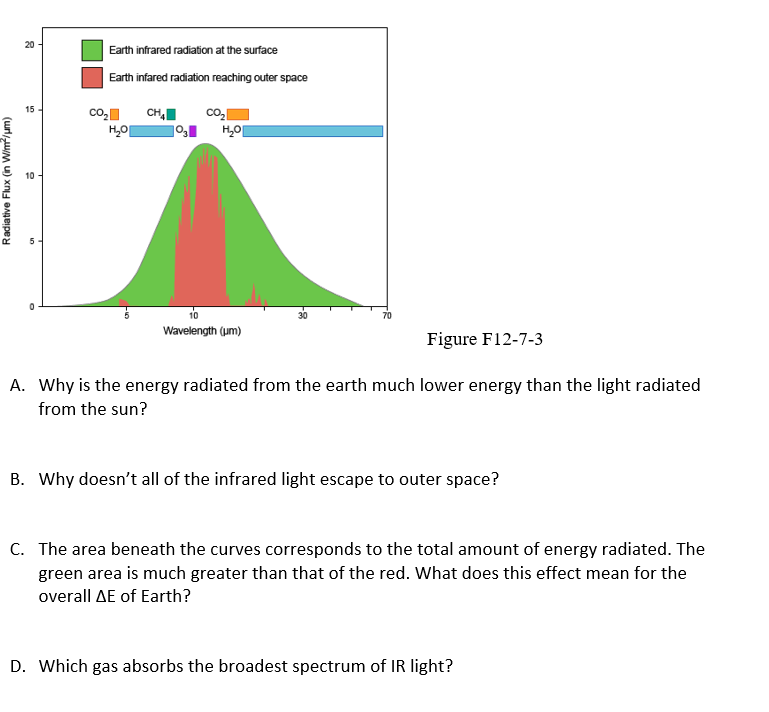
Transcribed Image Text:20
Earth infrared radiation at the surface
Earth infared radiation reaching outer space
15
co,
co,
CH,
HO
10
Wavelength (um)
Figure F12-7-3
A. Why is the energy radiated from the earth much lower energy than the light radiated
from the sun?
B. Why doesn't all of the infrared light escape to outer space?
C. The area beneath the curves corresponds to the total amount of energy radiated. The
green area is much greater than that of the red. What does this effect mean for the
overall AE of Earth?
(urluM u) xny angepe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning