200 200 190 190 180 180 Vapor 170 Temp oC 170 Liquid 160 160 150 150 140 140 50 25 Mole % Isoamyl Acetate . 100 Mole % Methyl Benzoate 75 50 75 100 25
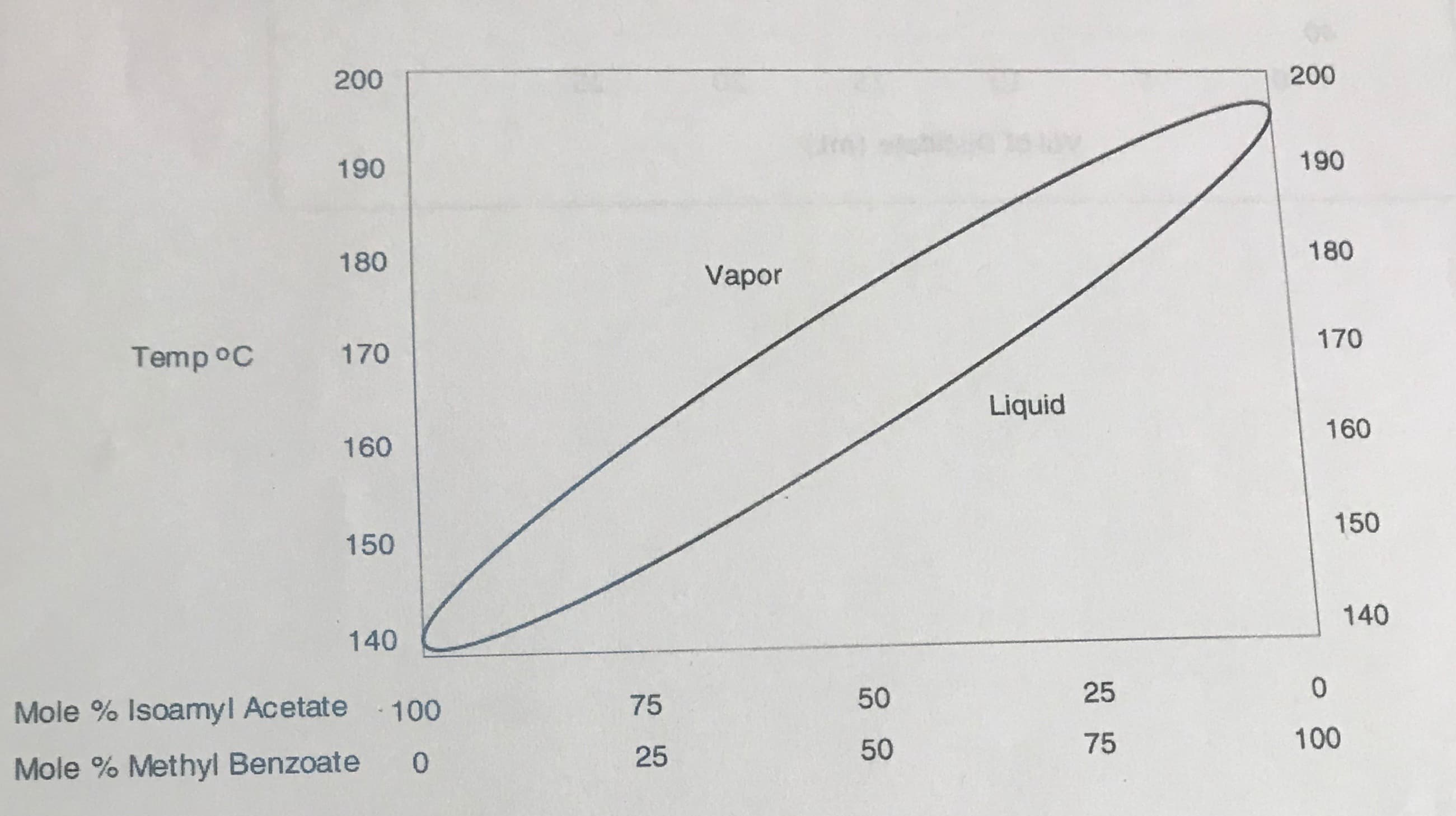
A mixture of 20 mL of isoamyl acetate (MW=130.2 g/mol and density= 0.88 g/mL) and 20 mL of methyl benzoate (MW= 136.2 g/mol and density =1.09 g/mL) is distilled. Calculate the mole percent for each component. Use these mole percents and the figure below to answer the following questions.
a. What is the initial boiling point of this mixture?
b. What is the composition of the vapor in equilibrium with the liquid? Is this composition the same as the composition of the initial condensate from simple distillation?
c. Instead of a simple distillation, you decide to use fractional distillation. Assuming two theoretical plates, what is the composition of the first fraction collected?
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images








