22. What is the standard enthalpy change for the reaction of lead(II) chloride with chlorine to give lead(IV) chloride? PBCI,(s) + Cl,(g) PBC1,(e) -- It is known that PbCl,(s) can be formed from the metal and Cl,(g), Pb(s) + Cl(g) PBCI,() AH = -359.4 kJ and that PbCl4E) can be formed directly from the clements. Pb(s) + 2 Cl,(g) PBCL,(E) AH = -329.3 kJ
22. What is the standard enthalpy change for the reaction of lead(II) chloride with chlorine to give lead(IV) chloride? PBCI,(s) + Cl,(g) PBC1,(e) -- It is known that PbCl,(s) can be formed from the metal and Cl,(g), Pb(s) + Cl(g) PBCI,() AH = -359.4 kJ and that PbCl4E) can be formed directly from the clements. Pb(s) + 2 Cl,(g) PBCL,(E) AH = -329.3 kJ
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 118QRT
Related questions
Question
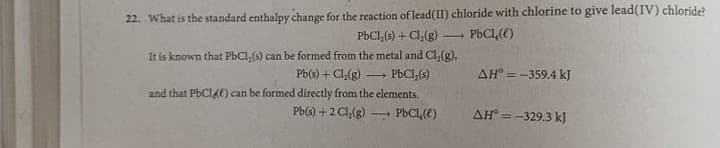
Transcribed Image Text:22. What is the standard enthalpy change for the reaction of lead(II) chloride with chlorine to give lead(IV) chloride?
PBC1,(5) + Cl,(g)
PbCl,(e)
It is known that PbCl,(s) can be formed from the metal and Cl,(g),
Pb(s) + Cl,(g)
PBCI,(s)
AH° = -359.4 kJ
and that PBCI4E) can be formed directly from the elements.
Pb(s) +2 Cl,(g)
PbCL,(E)
AH° = -329.3 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,