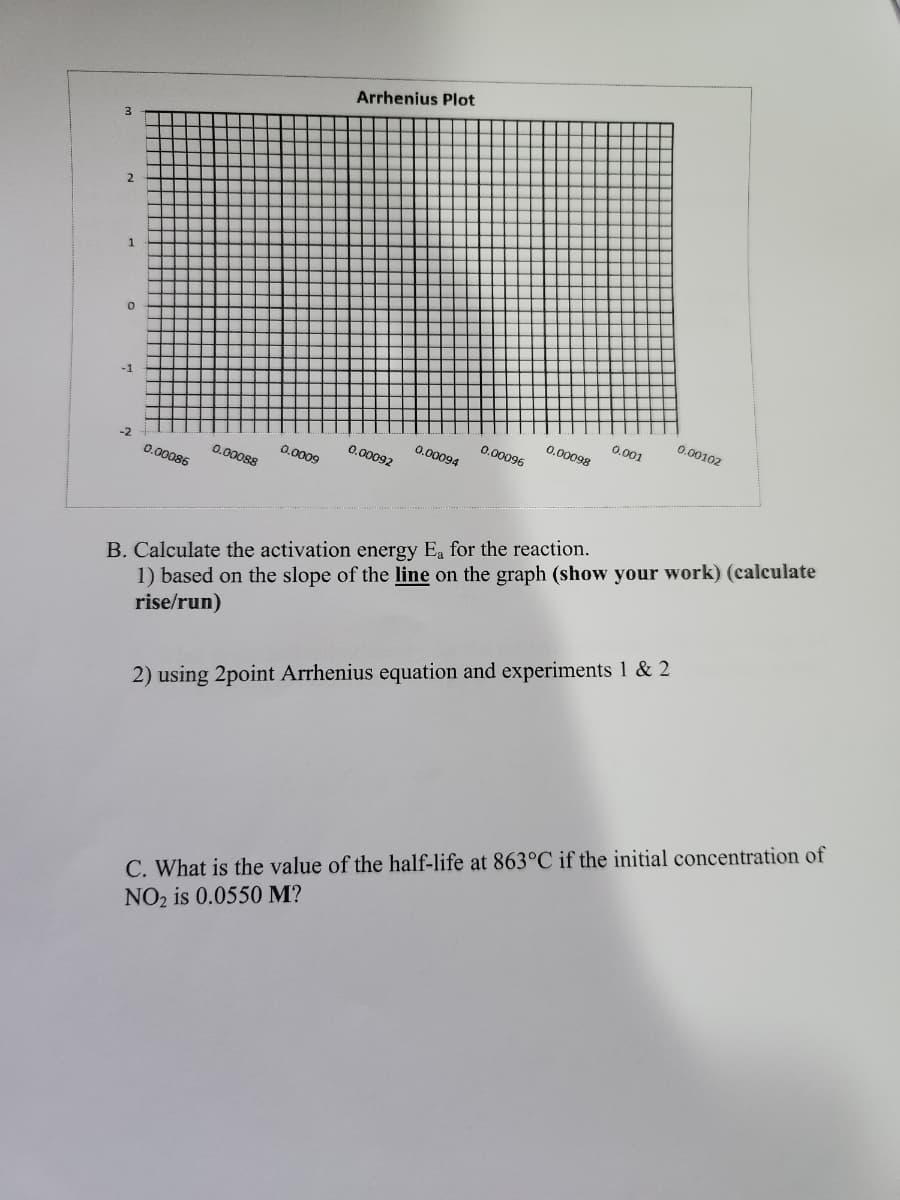
3 2 1 0 -1 -2 0.00086 0.00088 0.0009 Arrhenius Plot 0.00092 0.00094 0.00096 0.00098 0.001 0.00102 B. Calculate the activation energy Ea for the reaction. 1) based on the slope of the line on the graph (show your work) (calculate rise/run) 2) using 2point Arrhenius equation and experiments 1 & 2 C. What is the value of the half-life at 863°C if the initial concentration of NO₂ is 0.0550 M?
3 2 1 0 -1 -2 0.00086 0.00088 0.0009 Arrhenius Plot 0.00092 0.00094 0.00096 0.00098 0.001 0.00102 B. Calculate the activation energy Ea for the reaction. 1) based on the slope of the line on the graph (show your work) (calculate rise/run) 2) using 2point Arrhenius equation and experiments 1 & 2 C. What is the value of the half-life at 863°C if the initial concentration of NO₂ is 0.0550 M?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter13: Chemical Kinetics
Section: Chapter Questions
Problem 13.50QE
Related questions
Question
100%
Discc 16 On paper please
Transcribed Image Text:3
2
1
0
-1
-2
0.00086
0.00088
0.0009
Arrhenius Plot
0.00092
0.00094
0.00096
0.00098
0.001
0.00102
B. Calculate the activation energy Ea for the reaction.
1) based on the slope of the line on the graph (show your work) (calculate
rise/run)
2) using 2point Arrhenius equation and experiments 1 & 2
C. What is the value of the half-life at 863°C if the initial concentration of
NO₂ is 0.0550 M?

Transcribed Image Text:16. At higher temperatures, the same reaction: 2 NO₂+2 NO(g) + O₂(g)
follows second order kinetics.
Using some of the experimental data shown,
Temperature
k, M-¹ S-¹
Temperature
(K)
(°C)
863
780
727
11.59
2.34
0.380
A. Sketch an Arrhenius plot with properly labeled axes.
1/T
Ink
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
