3 Given that the density of KBr(s) is 2.75 g/cr cm° and that the length of an edge of a unit cell is 659 pm, determine how many formula units of KBr there are in a unit cell. formula units: The unit cells for NaCl and CsCl are shown. NaCl(s) CsCl(s) Based on your answer for the number of formula units of KBr(s) in a unit cell, how is the unit cell of KBr(s) likely to be structured? same as NaCl(s) same as CsCl(s) neither
3 Given that the density of KBr(s) is 2.75 g/cr cm° and that the length of an edge of a unit cell is 659 pm, determine how many formula units of KBr there are in a unit cell. formula units: The unit cells for NaCl and CsCl are shown. NaCl(s) CsCl(s) Based on your answer for the number of formula units of KBr(s) in a unit cell, how is the unit cell of KBr(s) likely to be structured? same as NaCl(s) same as CsCl(s) neither
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 23P
Related questions
Question
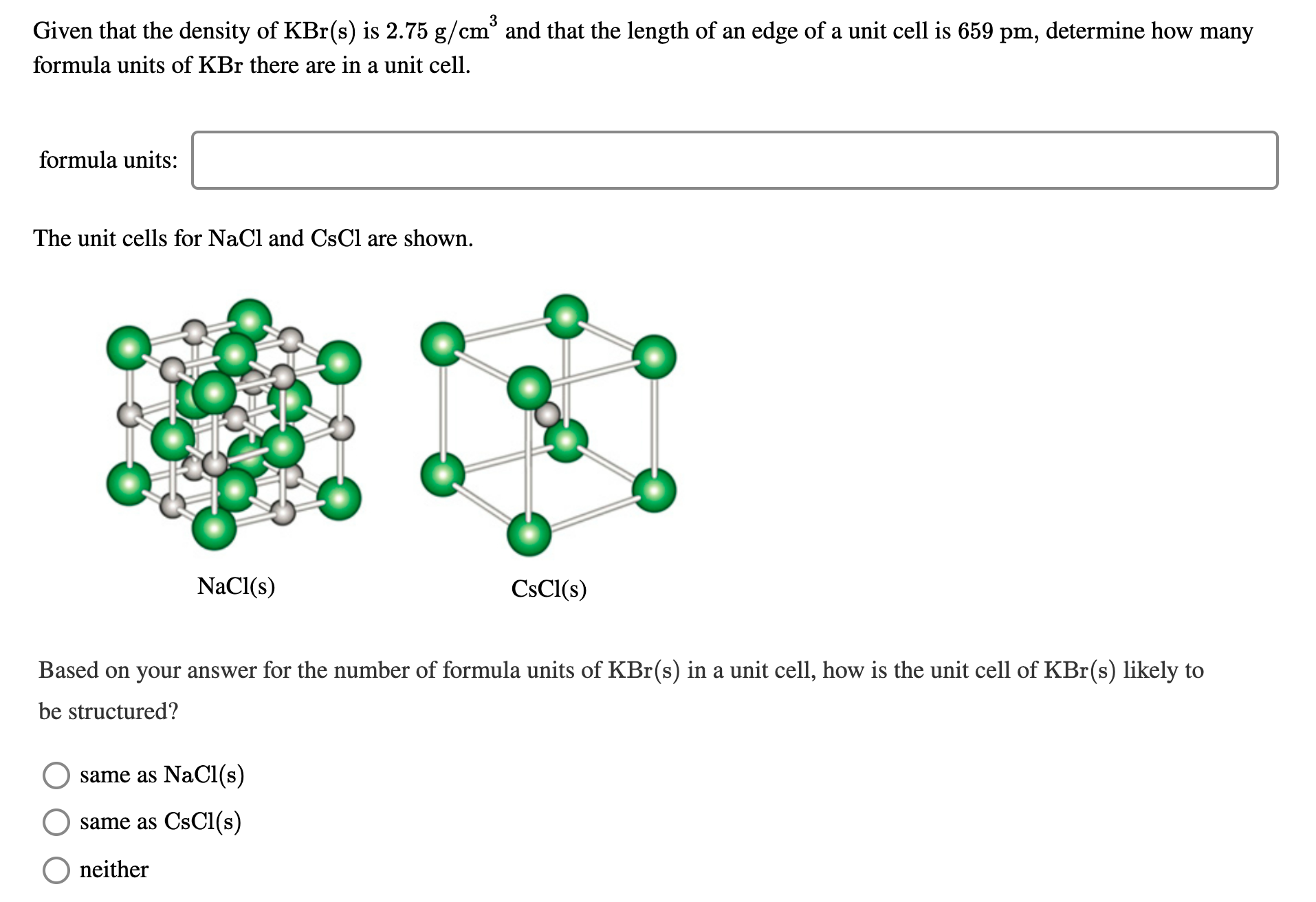
Transcribed Image Text:3
Given that the density of KBr(s) is 2.75 g/cr
cm° and that the length of an edge of a unit cell is 659 pm, determine how many
formula units of KBr there are in a unit cell.
formula units:
The unit cells for NaCl and CsCl are shown.
NaCl(s)
CsCl(s)
Based on your answer for the number of formula units of KBr(s) in a unit cell, how is the unit cell of KBr(s) likely to
be structured?
same as NaCl(s)
same as CsCl(s)
neither
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning