3. The half-life of 3P is t1/2 = 14.30 days. Suppose that mass of P is 32.00 amu. (a) Calculate the mass of 2P in 175.0-mg sample of Na35PO4 (b) How long does it take for the amount of 32P to decrease to 2.713 mg in this sample?
3. The half-life of 3P is t1/2 = 14.30 days. Suppose that mass of P is 32.00 amu. (a) Calculate the mass of 2P in 175.0-mg sample of Na35PO4 (b) How long does it take for the amount of 32P to decrease to 2.713 mg in this sample?
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.74E
Related questions
Question
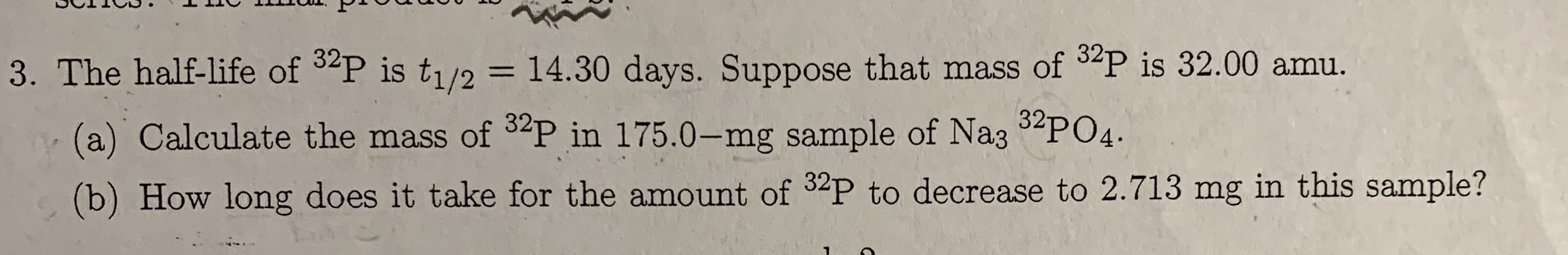
Transcribed Image Text:3. The half-life of 3P is t1/2 = 14.30 days. Suppose that mass of P is 32.00 amu.
(a) Calculate the mass of 2P in 175.0-mg sample of Na35PO4
(b) How long does it take for the amount of 32P to decrease to 2.713 mg in this sample?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning