3. Use formal charge to choose the best Lewis structure for CH;SOCH3. H :ö: H H :ö H a) H-C=S-C-H b) H-C-S-C-H н н H H :ö: H H :ö: H с) Н—С—S—С—н d) H-C=S-C-H H H H.
3. Use formal charge to choose the best Lewis structure for CH;SOCH3. H :ö: H H :ö H a) H-C=S-C-H b) H-C-S-C-H н н H H :ö: H H :ö: H с) Н—С—S—С—н d) H-C=S-C-H H H H.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 41QRT
Related questions
Question
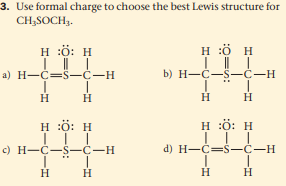
Transcribed Image Text:3. Use formal charge to choose the best Lewis structure for
CH;SOCH3.
H :ö: H
H :ö H
a) H-C=S-C-H
b) H-C-S-C-H
н н
H
H :ö: H
H :ö: H
с) Н—С—S—С—н
d) H-C=S-C-H
H
H
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning