If you are told that a certain metal element has a simple cubic unit cell structure, what additional information do you need in order calculate the atomic radius for that element? Select all correct answers. (You can assume that you already have the information necessary to do metric unit prefix conversions.)
Out of the following defects, which one must occur in pairs for ionic compounds with 1:1 stoichiometries?
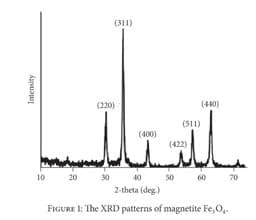
Consider this x-ray diffraction pattern for the mineral magnetite (Fe3O4) and Bragg's Law. Which of the following statements is true? (HINTs: Since all of these peaks result from the same x-ray scan, the same x-ray wavelength applies to all peaks in this pattern. You may also assume that the same order (n) applies to all peaks in this pattern.)
Step by step
Solved in 3 steps with 2 images








