Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 75AP: Phenols (ArOH) are relatively acidic, and the presence of a substituent group on the aromatic ring...
Related questions
Question
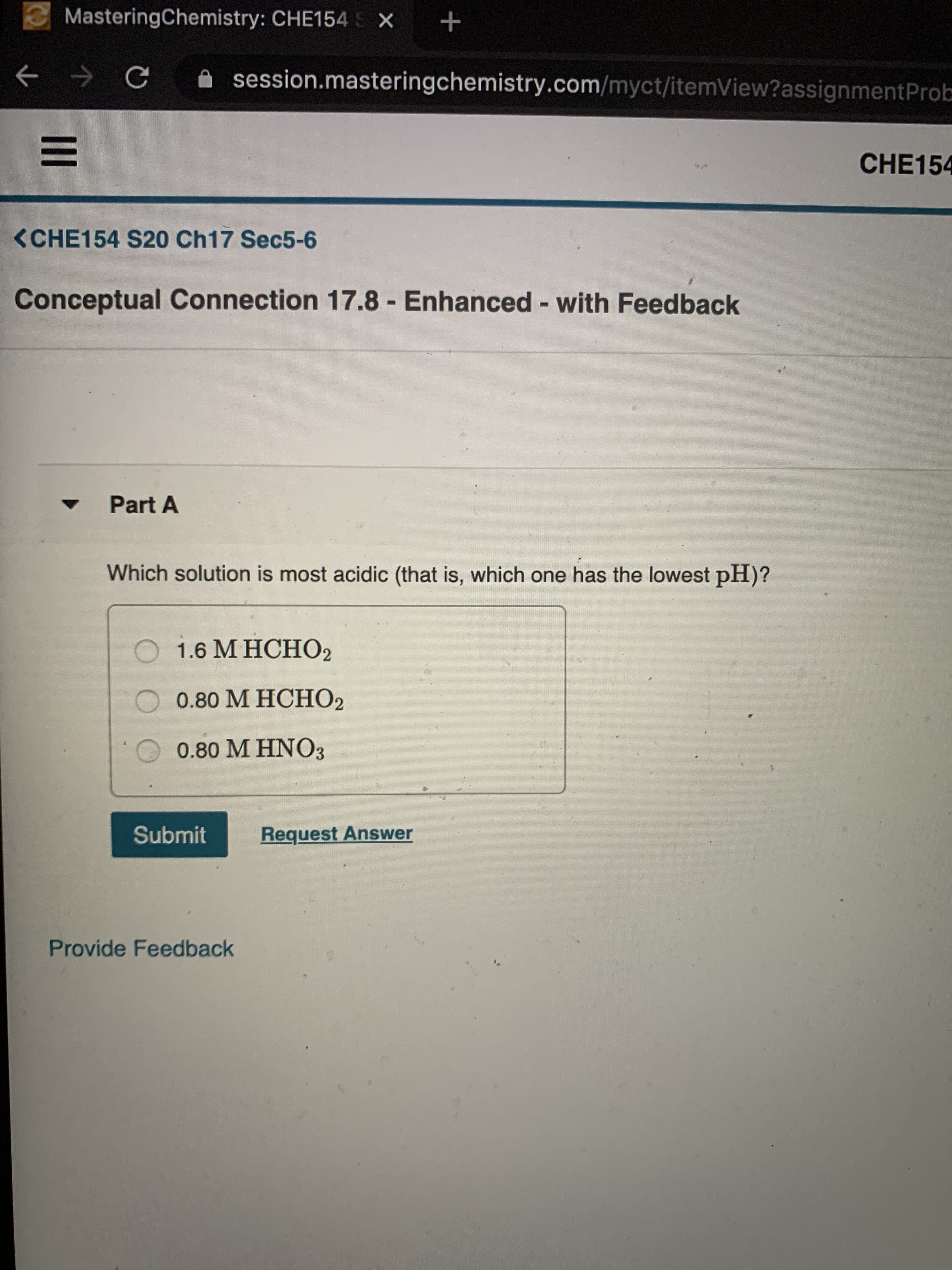
Transcribed Image Text:3MasteringChemistry: CHE154 S X
session.masteringchemistry.com/myct/itemView?assignmentProb
CHE154
<CHE154 S20 Ch17 Sec5-6
Conceptual Connection 17.8 - Enhanced - with Feedback
Part A
Which solution is most acidic (that is, which one has the lowest pH)?
О 1.6 МНСНО2
0.80 M HCHO2
0.80 M HNO3
Submit
Request Answer
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 9 images

Recommended textbooks for you

