4) Using heat capacities from the table and formation enthalpy of HCl(g) A¢H° = -92.3 kJ molª (at 298K), determine standard enthalpies of reaction Cl2 (g) + H2(g) => 2HC1 (g)) Assume that the heat capacities are independent of temperature. at 298K and 498K? Cp, JK-' mol-1 Gas Cl2 34 На НСІ 29 29
4) Using heat capacities from the table and formation enthalpy of HCl(g) A¢H° = -92.3 kJ molª (at 298K), determine standard enthalpies of reaction Cl2 (g) + H2(g) => 2HC1 (g)) Assume that the heat capacities are independent of temperature. at 298K and 498K? Cp, JK-' mol-1 Gas Cl2 34 На НСІ 29 29
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.12E: If the heat capacity varies withtemperature, abetter form ofequation 2.9 isto solve q=TiTfnCT-t A...
Related questions
Question
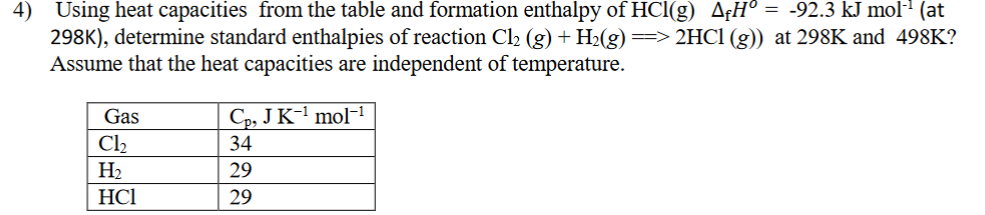
Transcribed Image Text:4)
Using heat capacities from the table and formation enthalpy of HCl(g) A¢H° = -92.3 kJ molª (at
298K), determine standard enthalpies of reaction Cl2 (g) + H2(g) => 2HC1 (g))
Assume that the heat capacities are independent of temperature.
at 298K and 498K?
Cp, JK-' mol-1
Gas
Cl2
34
На
НСІ
29
29
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning