4. 3) The data given in the table below is for the following reaction (run at 25°C): 2 NO + O, → 2 NO, 2(g) 2(g) Initial Concentration (M) Initial rate Experiment Mol/(L s) NO 02 0.0010 7 X 10-6 1 0.0010 0.0010 0.0020 14 X 10-6 0.0010 0.0030 21 X 106 0.0020 0.0030 84 X 10-6 0.0030 0.0030 189 X 10-6 1. Determine the orders of the reaction with respect to NO and O, (show all work): NO O2
4. 3) The data given in the table below is for the following reaction (run at 25°C): 2 NO + O, → 2 NO, 2(g) 2(g) Initial Concentration (M) Initial rate Experiment Mol/(L s) NO 02 0.0010 7 X 10-6 1 0.0010 0.0010 0.0020 14 X 10-6 0.0010 0.0030 21 X 106 0.0020 0.0030 84 X 10-6 0.0030 0.0030 189 X 10-6 1. Determine the orders of the reaction with respect to NO and O, (show all work): NO O2
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.68P
Related questions
Question
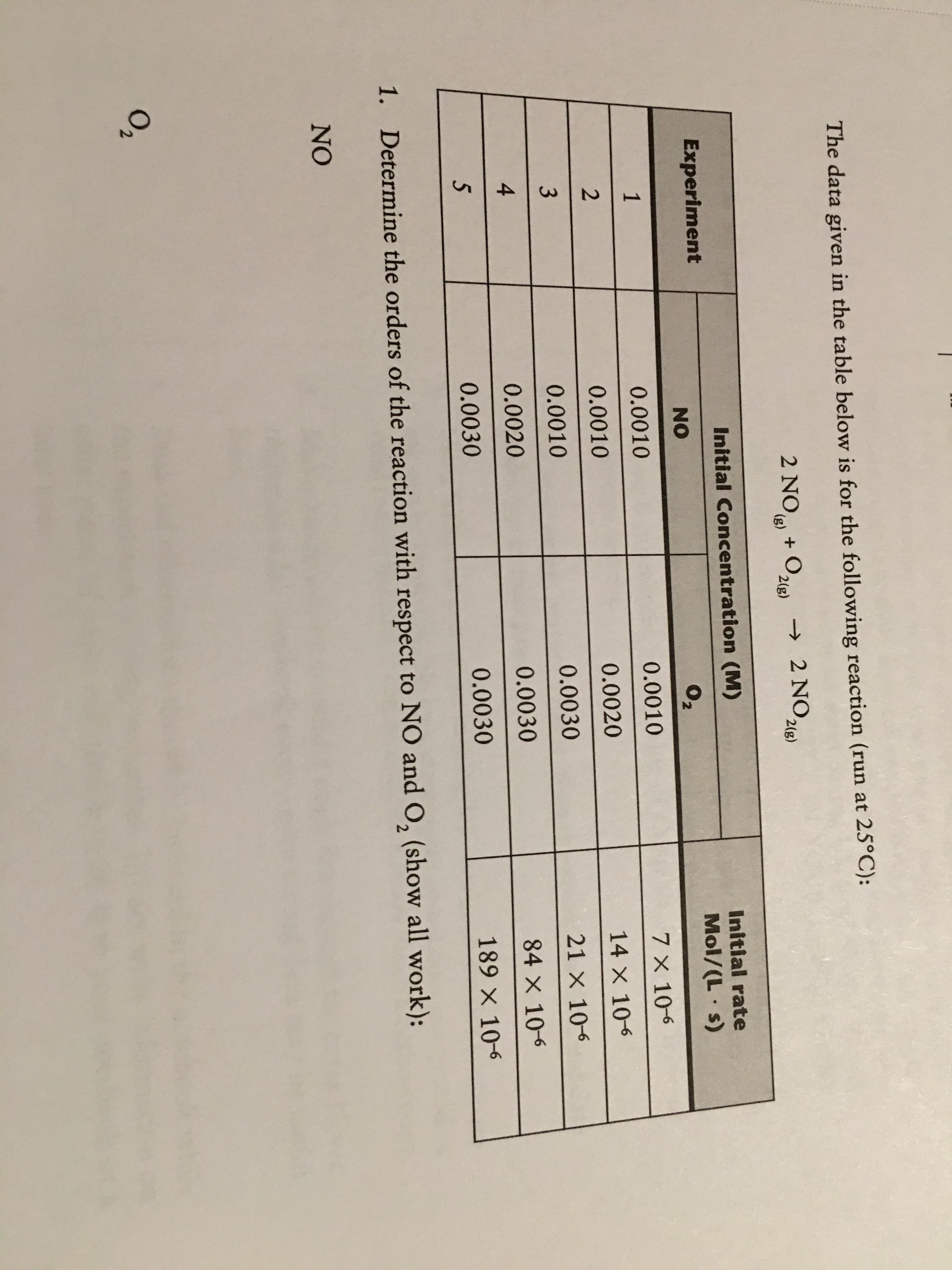
Transcribed Image Text:4.
3)
The data given in the table below is for the following reaction (run at 25°C):
2 NO + O,
→ 2 NO,
2(g)
2(g)
Initial Concentration (M)
Initial rate
Experiment
Mol/(L s)
NO
02
0.0010
7 X 10-6
1
0.0010
0.0010
0.0020
14 X 10-6
0.0010
0.0030
21 X 106
0.0020
0.0030
84 X 10-6
0.0030
0.0030
189 X 10-6
1.
Determine the orders of the reaction with respect to NO and O, (show all work):
NO
O2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning