4. a. The ¹H NMR spectrum of a compound with the molecular formula C-H₁0O2 is shown below (integral values are given above each set of peaks). Analysis of its ¹³C NMR spectrum shows peaks at 174, 61, 27, 14 and 9 ppm (see the posted lecture notes for June 7 for some guidance on ¹³C NMR spectroscopy). The IR spectrum shows a prominent peak at 1735 cm²¹ and no peaks with a stretching frequency above 3000 cm¹. What is the structure of this compound? 3.5 3.0 2.0 1.5
4. a. The ¹H NMR spectrum of a compound with the molecular formula C-H₁0O2 is shown below (integral values are given above each set of peaks). Analysis of its ¹³C NMR spectrum shows peaks at 174, 61, 27, 14 and 9 ppm (see the posted lecture notes for June 7 for some guidance on ¹³C NMR spectroscopy). The IR spectrum shows a prominent peak at 1735 cm²¹ and no peaks with a stretching frequency above 3000 cm¹. What is the structure of this compound? 3.5 3.0 2.0 1.5
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 48AP: The infrared spectrum of the compound with the mass spectrum shown below lacks any significant...
Related questions
Question
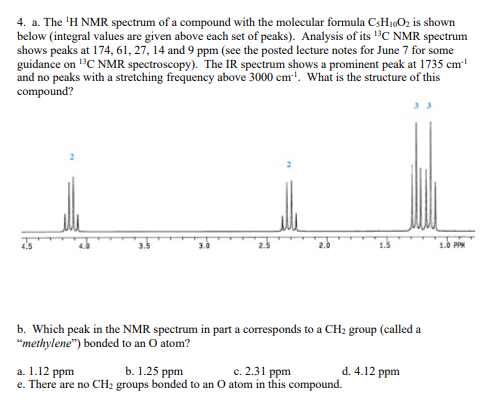
Transcribed Image Text:4. a. The ¹H NMR spectrum of a compound with the molecular formula C3H₁0O₂ is shown
below (integral values are given above each set of peaks). Analysis of its ¹3C NMR spectrum
shows peaks at 174, 61, 27, 14 and 9 ppm (see the posted lecture notes for June 7 for some
guidance on ¹³C NMR spectroscopy). The IR spectrum shows a prominent peak at 1735 cm¹¹
and no peaks with a stretching frequency above 3000 cm³¹. What is the structure of this
compound?
3.0
b. Which peak in the NMR spectrum in part a corresponds to a CH₂ group (called a
"methylene") bonded to an O atom?
a. 1.12 ppm
b. 1.25 ppm
c. 2.31 ppm
e. There are no CH₂ groups bonded to an O atom in this compound.
d. 4.12 ppm
1.0 PPM
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole