4. Deducing quaternary structure via SDS-PAGE. SDS-PAGE is a convenient method for separating polypeptides solely on the basis of size. Small polypeptides travel faster than large ones; rate of migration through the gel is inversely proportional to the logarithm of molecular weight. The subunit structure of a multimeric protein often can be deduced using this technique in conjunction with a protein cross-linking agent. Cross-linking agents react with amino acid residues of two polypeptides that are in contact, thereby linking them by covalent bonds. After limited treatment with the reagent, so that some but not all subunits become cross-linked to their neighbors, the protein is subjected to SDS-PAGE and the molecular weights of the resulting proteins (bands) are estimated. The results of such experiments on two proteins (Protein A and Protein B) are shown below. What is the most likely subunit structure of each protein? Protein A SDS-PAGE cross-linking agent MW 105,000 70,000 35,000 Protein B SDS-PAGE cross-linking agent MW 55,000 40,000 15,000
4. Deducing quaternary structure via SDS-PAGE. SDS-PAGE is a convenient method for separating polypeptides solely on the basis of size. Small polypeptides travel faster than large ones; rate of migration through the gel is inversely proportional to the logarithm of molecular weight. The subunit structure of a multimeric protein often can be deduced using this technique in conjunction with a protein cross-linking agent. Cross-linking agents react with amino acid residues of two polypeptides that are in contact, thereby linking them by covalent bonds. After limited treatment with the reagent, so that some but not all subunits become cross-linked to their neighbors, the protein is subjected to SDS-PAGE and the molecular weights of the resulting proteins (bands) are estimated. The results of such experiments on two proteins (Protein A and Protein B) are shown below. What is the most likely subunit structure of each protein? Protein A SDS-PAGE cross-linking agent MW 105,000 70,000 35,000 Protein B SDS-PAGE cross-linking agent MW 55,000 40,000 15,000
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter31: Completing The Protein Life Cycle: Folding, Processing, And Degradation
Section: Chapter Questions
Problem 3P: Understanding the Relevance of Chaperones in Protein Folding Protein molecules, like all molecules,...
Related questions
Question
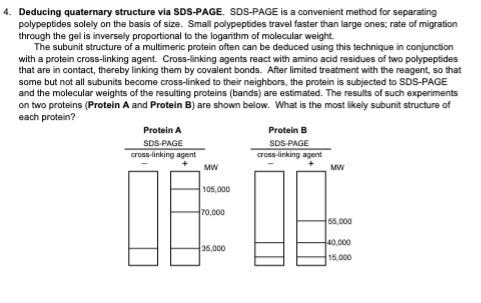
Transcribed Image Text:4. Deducing quaternary structure via SDS-PAGE. SDS-PAGE is a convenient method for separating
polypeptides solely on the basis of size. Small polypeptides travel faster than large ones; rate of migration
through the gel is inversely proportional to the logarithm of molecular weight.
The subunit structure of a multimeric protein often can be deduced using this technique in conjunction
with a protein cross-linking agent. Cross-linking agents react with amino acid residues of two polypeptides
that are in contact, thereby linking them by covalent bonds. After limited treatment with the reagent, so that
some but not all subunits become cross-linked to their neighbors, the protein is subjected to SDS-PAGE
and the molecular weights of the resulting proteins (bands) are estimated. The results of such experiments
on two proteins (Protein A and Protein B) are shown below. What is the most likely subunit structure of
each protein?
Protein A
SDS-PAGE
cross-linking agent
MW
105,000
70,000
35,000
Protein B
SDS-PAGE
cross-linking agent
MW
55,000
40,000
15,000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax