4. Let us consider a process flow diagram describing the water conditioning setup for a salt- water fish tank. This process removes all of the dirt from the inlet stream and adds oxygen. and salt to a final salt-to-water ratio of 0.0375, and an oxygen-to-water ratio of 0.0015. The inlet stream, which contains water, salt, dissolved oxygen, and dirt enters a sedimentation unit. This unit concentrates all of the dirt and removes it at a concentration of 20.0 wt% dirt. This dirt is then subsequently filtered out of the system. While the concentrating of dirt is the only separation achieved in the sedimentation unit, it also creates two additional outlet streams, which feed the oxygenator and the salinator. In the oxygenator, oxygen is bubbled into the water to achieve an oxygen-to-water ratio of 0.0020. In the salinator, salt tablets are dissolved into the stream as needed. Wigh 400 In S 9 20 Isse Ⓒ100% W
4. Let us consider a process flow diagram describing the water conditioning setup for a salt- water fish tank. This process removes all of the dirt from the inlet stream and adds oxygen. and salt to a final salt-to-water ratio of 0.0375, and an oxygen-to-water ratio of 0.0015. The inlet stream, which contains water, salt, dissolved oxygen, and dirt enters a sedimentation unit. This unit concentrates all of the dirt and removes it at a concentration of 20.0 wt% dirt. This dirt is then subsequently filtered out of the system. While the concentrating of dirt is the only separation achieved in the sedimentation unit, it also creates two additional outlet streams, which feed the oxygenator and the salinator. In the oxygenator, oxygen is bubbled into the water to achieve an oxygen-to-water ratio of 0.0020. In the salinator, salt tablets are dissolved into the stream as needed. Wigh 400 In S 9 20 Isse Ⓒ100% W
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
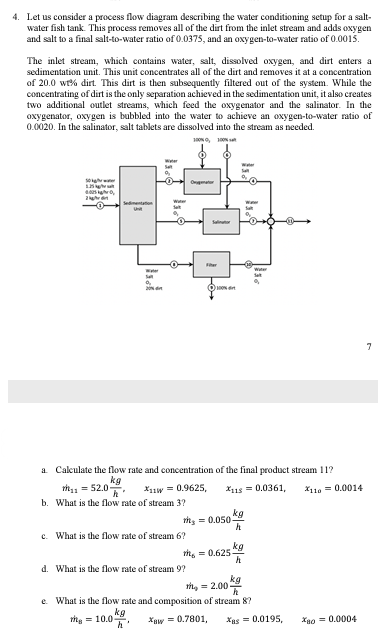
Transcribed Image Text:4. Let us consider a process flow diagram describing the water conditioning setup for a salt-
water fish tank. This process removes all of the dirt from the inlet stream and adds oxygen
and salt to a final salt-to-water ratio of 0.0375, and an oxygen-to-water ratio of 0.0015.
The inlet stream, which contains water, salt, dissolved oxygen, and dirt enters a
sedimentation unit. This unit concentrates all of the dirt and removes it at a concentration
of 20.0 wt% dirt. This dirt is then subsequently filtered out of the system. While the
concentrating of dirt is the only separation achieved in the sedimentation unit, it also creates
two additional outlet streams, which feed the oxygenator and the salinator. In the
oxygenator, oxygen is bubbled into the water to achieve an oxygen-to-water ratio of
0.0020. In the salinator, salt tablets are dissolved into the stream as needed.
Sa
20
O
Ogator
c. What is the flow rate of stream 6?
Ser
*11W = 0.9625,
a. Calculate the flow rate and concentration of the final product stream 11?
kg
t₁ = 52.0
*115 = 0.0361,
*110 = 0.0014
b. What is the flow rate of stream 3?
d. What is the flow rate of stream 9?
Ⓒ100%
th=0.050-
kg
h
kg
h
th=0.625-
kg
9
the 2.003
00
e. What is the flow rate and composition of stream 8?
kg
the = 10.0
Xaw = 0.7801, Xas = 0.0195,
*ao = 0.0004
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The