4. Metallurgy in the Auto Industry The following illustration appeared in a newspaper article (New York Times: Science Times September 15, 2009) about innovation in the American steel industry. The article outlined efforts to make strong, lightweight steel for cars. heat, C col ferrite austenite martensite iron carbon carbon Note the cool names for the two alloys, named after a famous British sports car maker. a) What is the packing system for the iron atoms in ferrite? b) Heating the ferrite in the presence of carbon leads to formation of austenite. What is the packing system for the iron atoms in austenite? c) What sort of coordination environment does the carbon occupy in austenite? d) What fraction of these holes are occupied? e) Given this unit cell, what is the ratio of carbon to iron in austenite? f) Careful cooling of the austenite leads to formation of martensite. How would you describe the coordination environment of the carbon in martensite? Note: This is not one of the three coordination geometries discussed. Describe it to the best of your ability. g) If the ratio of iron to carbon in a sample of martensite is 20:1, what does that tell you about the frequency with which carbon atoms occur in martensite unit cells?
4. Metallurgy in the Auto Industry The following illustration appeared in a newspaper article (New York Times: Science Times September 15, 2009) about innovation in the American steel industry. The article outlined efforts to make strong, lightweight steel for cars. heat, C col ferrite austenite martensite iron carbon carbon Note the cool names for the two alloys, named after a famous British sports car maker. a) What is the packing system for the iron atoms in ferrite? b) Heating the ferrite in the presence of carbon leads to formation of austenite. What is the packing system for the iron atoms in austenite? c) What sort of coordination environment does the carbon occupy in austenite? d) What fraction of these holes are occupied? e) Given this unit cell, what is the ratio of carbon to iron in austenite? f) Careful cooling of the austenite leads to formation of martensite. How would you describe the coordination environment of the carbon in martensite? Note: This is not one of the three coordination geometries discussed. Describe it to the best of your ability. g) If the ratio of iron to carbon in a sample of martensite is 20:1, what does that tell you about the frequency with which carbon atoms occur in martensite unit cells?
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter11: Intermolecular Forces And Liquids
Section: Chapter Questions
Problem 39IL
Related questions
Question
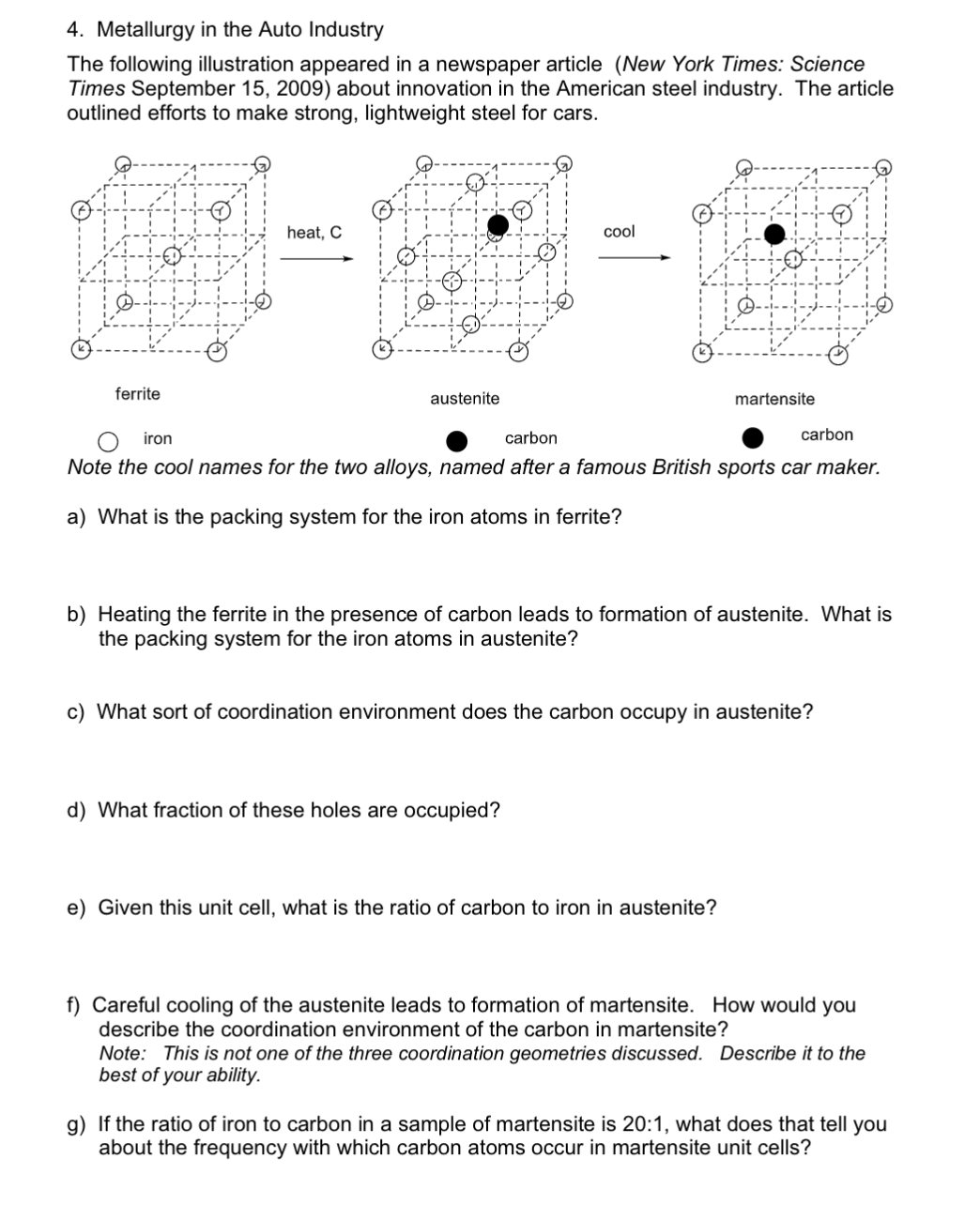
Transcribed Image Text:4. Metallurgy in the Auto Industry
The following illustration appeared in a newspaper article (New York Times: Science
Times September 15, 2009) about innovation in the American steel industry. The article
outlined efforts to make strong, lightweight steel for cars.
heat, C
cool
ferrite
austenite
martensite
iron
carbon
carbon
Note the cool names for the two alloys, named after a famous British sports car maker.
a) What is the packing system for the iron atoms in ferrite?
b) Heating the ferrite in the presence of carbon leads to formation of austenite. What is
the packing system for the iron atoms in austenite?
c) What sort of coordination environment does the carbon occupy in austenite?
d) What fraction of these holes are occupied?
e) Given this unit cell, what is the ratio of carbon to iron in austenite?
f) Careful cooling of the austenite leads to formation of martensite. How would you
describe the coordination environment of the carbon in martensite?
Note: This is not one of the three coordination geometries discussed. Describe it to the
best of your ability.
g) If the ratio of iron to carbon in a sample of martensite is 20:1, what does that tell you
about the frequency with which carbon atoms occur in martensite unit cells?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
