5. An experiment is performed to measure the mass percent of Caco,(s) in eggshells. Five different samples of Caco,(s) of known mass react with an excess of 2.0M HCI(aq) in identical sealed, rigid reaction vessels. The pressure of the gas produced is measured with a pressure gauge attached to the reaction vessel. Since the reaction is exothermic, the reaction system is cooled to its original temperature before Cooling the HCl(aq) to a lower temperature than it was in the original experiment the pressure is recorded. The experimental data are used to create the calibration line below. Using eggshells that are more finely powdered than those used in the original experiment 0.10- E 0.09- 0.08- Using 2.0M CH,COOH(aq) instead of 2.0M HCl(aq) 0.07- 0.06- 5 0.05- 0.04 E0.03- Reducing the volume of the reaction vessel CLEAR ALL 0.02 os ++++ ++++ ++ 0.15 0.10 Mass of CaCo,(s) (grams) 0.20 The experiment is repeated with an eggshell sample, and the experimental data are recorded in the table below. Mass of eggshell sample 0.200g Pressure prior to reaction 0.800atm Pressure at completion of reaction 0.870atm
5. An experiment is performed to measure the mass percent of Caco,(s) in eggshells. Five different samples of Caco,(s) of known mass react with an excess of 2.0M HCI(aq) in identical sealed, rigid reaction vessels. The pressure of the gas produced is measured with a pressure gauge attached to the reaction vessel. Since the reaction is exothermic, the reaction system is cooled to its original temperature before Cooling the HCl(aq) to a lower temperature than it was in the original experiment the pressure is recorded. The experimental data are used to create the calibration line below. Using eggshells that are more finely powdered than those used in the original experiment 0.10- E 0.09- 0.08- Using 2.0M CH,COOH(aq) instead of 2.0M HCl(aq) 0.07- 0.06- 5 0.05- 0.04 E0.03- Reducing the volume of the reaction vessel CLEAR ALL 0.02 os ++++ ++++ ++ 0.15 0.10 Mass of CaCo,(s) (grams) 0.20 The experiment is repeated with an eggshell sample, and the experimental data are recorded in the table below. Mass of eggshell sample 0.200g Pressure prior to reaction 0.800atm Pressure at completion of reaction 0.870atm
Chapter5: Gases
Section: Chapter Questions
Problem 165MP
Related questions
Question
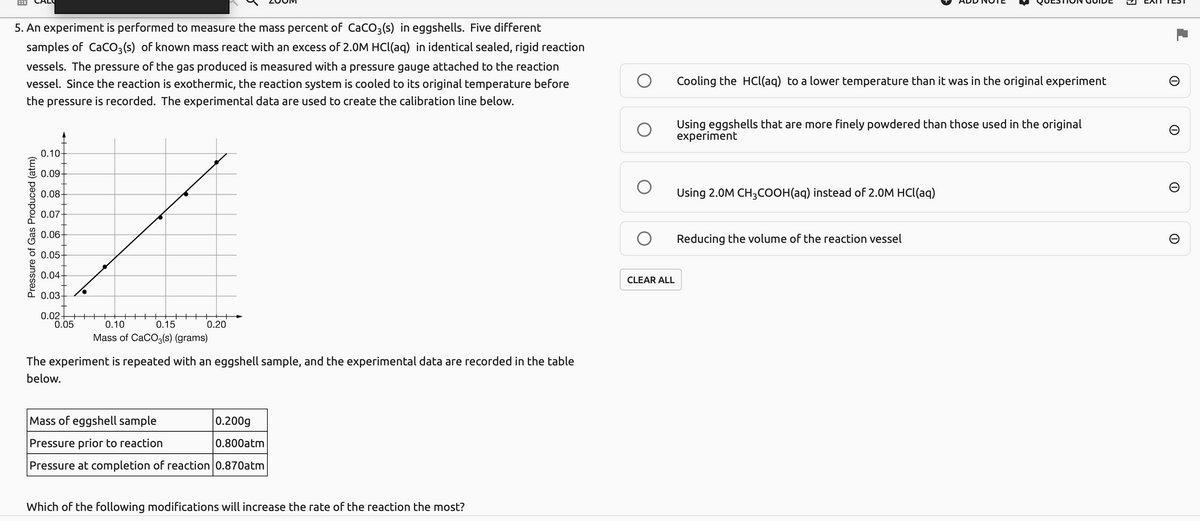
Transcribed Image Text:5. An experiment is performed to measure the mass percent of CaCO3(s) in eggshells. Five different
samples of CaCO3(s) of known mass react with an excess of 2.0M HCl(aq) in identical sealed, rigid reaction
vessels. The pressure of the gas produced is measured with a pressure gauge attached to the reaction
vessel. Since the reaction is exothermic, the reaction system is cooled to its original temperature before
Cooling the HCl(aq) to a lower temperature than it was in the original experiment
the pressure is recorded. The experimental data are used to create the calibration line below.
Using eggshells that are more finely powdered than those used in the original
experiment
0.10-
0.09
0.08-
Using 2.0M CH3COOH(aq) instead of 2.0M HCl(aq)
0.07
* 0.06-
Reducing the volume of the reaction vessel
0.05
0.04
CLEAR ALL
0.03-
0.02-
0.05
0.10
0.15
0.20
Mass of CaCO3(s) (grams)
The experiment is repeated with an eggshell sample, and the experimental data are recorded in the table
below.
Mass of eggshell sample
|0.200g
Pressure prior to reaction
0.800atm
Pressure at completion of reaction 0.870atm
Which of the following modifications will increase the rate of the reaction the most?
Pressure of Gas Produced (atm)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning