5. Aqueous solutions of AX, BY, and BZ (where A and B are cations; and X, Y, and Z are anions) are mixed together with the following results: AX(aq)+BY(aq)no precipitate AX(aq)+ BZ(aq)precipitate Which of the following choices is insoluble in water? b. AY d. BY c. BX a. AZ
5. Aqueous solutions of AX, BY, and BZ (where A and B are cations; and X, Y, and Z are anions) are mixed together with the following results: AX(aq)+BY(aq)no precipitate AX(aq)+ BZ(aq)precipitate Which of the following choices is insoluble in water? b. AY d. BY c. BX a. AZ
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 61E: A 100.0-mL aliquot of 0.200 M aqueous potassium hydroxide is mixed with 100.0 mL of 0.200 M aqueous...
Related questions
Question
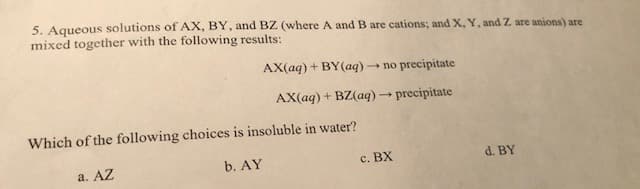
Transcribed Image Text:5. Aqueous solutions of AX, BY, and BZ (where A and B are cations; and X, Y, and Z are anions) are
mixed together with the following results:
AX(aq)+BY(aq)no precipitate
AX(aq)+ BZ(aq)precipitate
Which of the following choices is insoluble in water?
b. AY
d. BY
c. BX
a. AZ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
