5. I The biochemistry that takes place inside cells depends on O various elements, such as sodium, potassium, and calcium, that are dissolved in water as ions. These ions enter cells through nar- row pores in the cell membrane known as ion channels. Each ion channel, which is formed from a specialized protein molecule, is selective for one type of ion. Measurements with microelectrodes have shown that a 0.30-nm-diameter potassium ion (K+) channel carries a current of 1.8 pA. a. How many potassium ions pass through if the ion channel opens for 1.0 ms?
5. I The biochemistry that takes place inside cells depends on O various elements, such as sodium, potassium, and calcium, that are dissolved in water as ions. These ions enter cells through nar- row pores in the cell membrane known as ion channels. Each ion channel, which is formed from a specialized protein molecule, is selective for one type of ion. Measurements with microelectrodes have shown that a 0.30-nm-diameter potassium ion (K+) channel carries a current of 1.8 pA. a. How many potassium ions pass through if the ion channel opens for 1.0 ms?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.12QAP
Related questions
Question
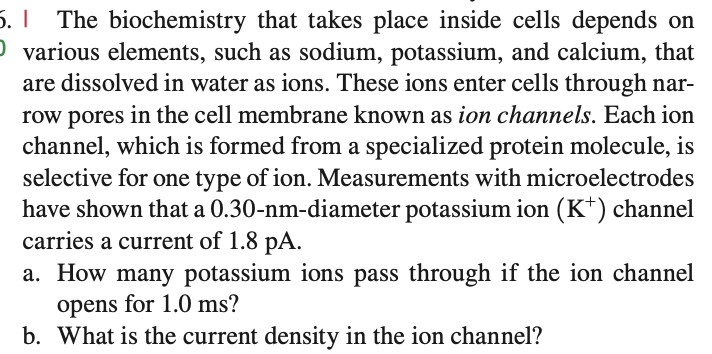
Transcribed Image Text:5. | The biochemistry that takes place inside cells depends on
O various elements, such as sodium, potassium, and calcium, that
are dissolved in water as ions. These ions enter cells through nar-
row pores in the cell membrane known as ion channels. Each ion
channel, which is formed from a specialized protein molecule, is
selective for one type of ion. Measurements with microelectrodes
have shown that a 0.30-nm-diameter potassium ion (K+) channel
carries a current of 1.8 pA.
a. How many potassium ions pass through if the ion channel
opens for 1.0 ms?
b. What is the current density in the ion channel?
Expert Solution
Step 1
Explanation
Since we know from the current and charge relation
q = I x t..................(i)
where q is charge and t is time in sec
And also charge is a given as
q = n x e............(ii)
where n is number of charged particle and e is electronic charge. The value of e is 1.6 x 10-19 C
From eq (i) and eq (ii)
n x e = I x t
Hence n = (I x t) / e.............(iii)
Therefore number of K+ can be calculated using eq (iii)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

