5. The table below shows the elements in the third period of the Periodic Table, the number of electrons in their outer energy level, their oxidation state in their common compounds and their melting points. element Na Mg Al Si CI Ar number of outer electrons 1 3 oxidation state +1 +2 +3 +4/-4 -3 -2 -1 01 melting point/C 98 650 660 1414 317 115 -101 -189 Describe why sillicon have significantly high melting point The no of outer electron will affect the effective nuclear charge. Explain how effective nuclear charge can cause the decreasing the atomic size Explain why Na, Mg and Al are good conductors of electricity. Which element exists as diatomic molecules of the type X2? l) ilI) Iv) 7, P. 4.
5. The table below shows the elements in the third period of the Periodic Table, the number of electrons in their outer energy level, their oxidation state in their common compounds and their melting points. element Na Mg Al Si CI Ar number of outer electrons 1 3 oxidation state +1 +2 +3 +4/-4 -3 -2 -1 01 melting point/C 98 650 660 1414 317 115 -101 -189 Describe why sillicon have significantly high melting point The no of outer electron will affect the effective nuclear charge. Explain how effective nuclear charge can cause the decreasing the atomic size Explain why Na, Mg and Al are good conductors of electricity. Which element exists as diatomic molecules of the type X2? l) ilI) Iv) 7, P. 4.
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 131AE
Related questions
Question
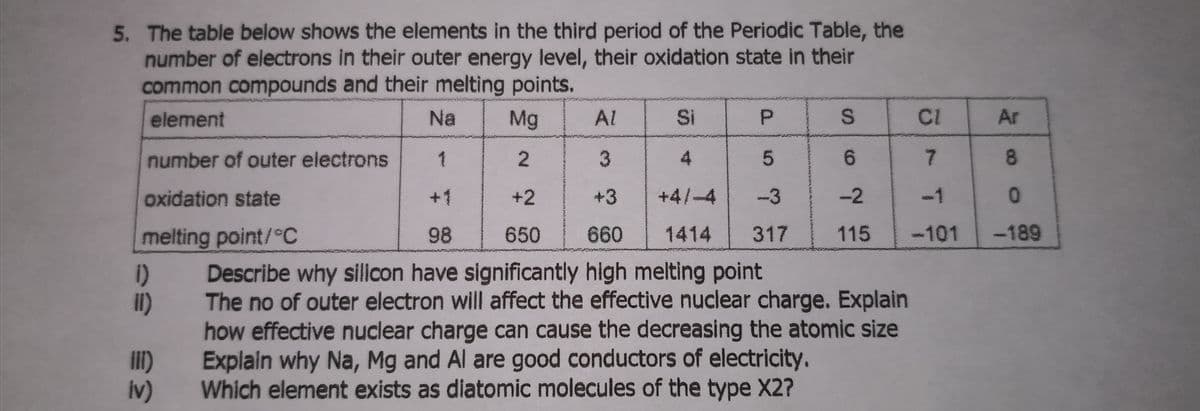
Transcribed Image Text:5. The table below shows the elements in the third period of the Periodic Table, the
number of electrons in their outer energy level, their oxidation state in their
common compounds and their melting points.
element
Na
Mg
Al
Si
P.
CI
Ar
number of outer electrons
1
2.
4
7.
8.
oxidation state
+1
+2
+3
+4/-4
-3
-2
-1
melting point/°C
98
650
660
1414
317
115
-101
-189
Describe why silicon have significantly high melting point
1)
The no of outer electron will affect the effective nuclear charge. Explain
il)
ilI)
Iv)
how effective nuclear charge can cause the decreasing the atomic size
Explain why Na, Mg and Al are good conductors of electricity.
Which element exists as diatomic molecules of the type X2?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning