557571&snapshotld=1324548&id =5636490008takeld 4bfba59b09f0e5b& Q Search this course X References squestion. Use the References to access important values if needed Consider the following reaction: H 1000 NH3(g) + HI(g) NH4I(s) A-Z If a flask maintained at 686 K contains 0.132 moles of NH I(s) in equilibrium with 9.50x10 MHI(g), what is the value of the equilbrium constant at 686 K? M NH3 (g) and 7.45x10 K = Submit Answer Regenerate Group Calculate K from Equilibrium Concentrations: This is group attempt 1 of 5 Next Autosaved at 12:59 PM Back 1:05 PM 11 9/24/2019 hp **
557571&snapshotld=1324548&id =5636490008takeld 4bfba59b09f0e5b& Q Search this course X References squestion. Use the References to access important values if needed Consider the following reaction: H 1000 NH3(g) + HI(g) NH4I(s) A-Z If a flask maintained at 686 K contains 0.132 moles of NH I(s) in equilibrium with 9.50x10 MHI(g), what is the value of the equilbrium constant at 686 K? M NH3 (g) and 7.45x10 K = Submit Answer Regenerate Group Calculate K from Equilibrium Concentrations: This is group attempt 1 of 5 Next Autosaved at 12:59 PM Back 1:05 PM 11 9/24/2019 hp **
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.12QAP
Related questions
Question
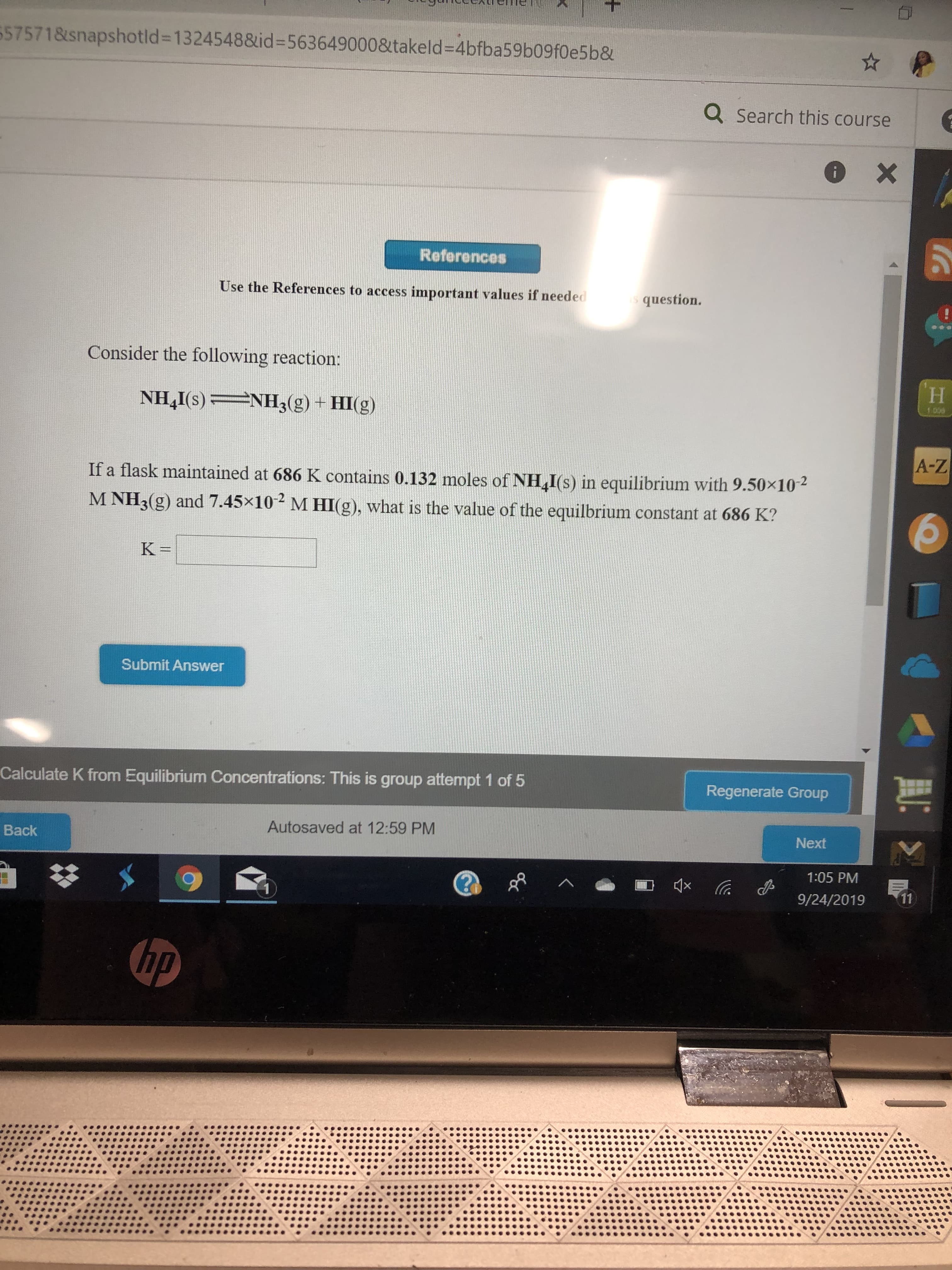
Transcribed Image Text:557571&snapshotld=1324548&id =5636490008takeld 4bfba59b09f0e5b&
Q Search this course
X
References
squestion.
Use the References to access important values if needed
Consider the following reaction:
H
1000
NH3(g) + HI(g)
NH4I(s)
A-Z
If a flask maintained at 686 K contains 0.132 moles of NH I(s) in equilibrium with 9.50x10
MHI(g), what is the value of the equilbrium constant at 686 K?
M NH3 (g) and 7.45x10
K =
Submit Answer
Regenerate Group
Calculate K from Equilibrium Concentrations: This is group attempt 1 of 5
Next
Autosaved at 12:59 PM
Back
1:05 PM
11
9/24/2019
hp
**
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning