575718snapshotld=1324548& id-563649007& Q Search this course Reference Use the References to access important va eeded for this question. The equilibrium constant, K, for the following reaction is 1.31x102 at 502 K. PCI5(g) PCl3(g)+ C2(g) An equilibrium mixture of the three gases in a 18.3 L container at 502 K contains 0.161 M PCls, 4.59x10 M PCI3 and 4.59x102 M Cl,. What will be the concentrations of the three gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at constant temperature to a volume of 7.43 L? [PCI5]= [PCI3]= [Cl2] Disturbing Equilibrium Volumes (Quantitative): This is group attempt 1 of 5 Next Back 1:35 PM 9/24/2019 hp X
575718snapshotld=1324548& id-563649007& Q Search this course Reference Use the References to access important va eeded for this question. The equilibrium constant, K, for the following reaction is 1.31x102 at 502 K. PCI5(g) PCl3(g)+ C2(g) An equilibrium mixture of the three gases in a 18.3 L container at 502 K contains 0.161 M PCls, 4.59x10 M PCI3 and 4.59x102 M Cl,. What will be the concentrations of the three gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at constant temperature to a volume of 7.43 L? [PCI5]= [PCI3]= [Cl2] Disturbing Equilibrium Volumes (Quantitative): This is group attempt 1 of 5 Next Back 1:35 PM 9/24/2019 hp X
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.40QE
Related questions
Question
![575718snapshotld=1324548& id-563649007&
Q Search this course
Reference
Use the References to access important va
eeded for this question.
The equilibrium constant, K, for the following reaction is 1.31x102 at 502 K.
PCI5(g) PCl3(g)+ C2(g)
An equilibrium mixture of the three gases in a 18.3 L container at 502 K contains 0.161 M
PCls, 4.59x10 M PCI3 and 4.59x102 M Cl,. What will be the concentrations of the three
gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at
constant temperature to a volume of 7.43 L?
[PCI5]=
[PCI3]=
[Cl2]
Disturbing Equilibrium Volumes (Quantitative): This is group attempt 1 of 5
Next
Back
1:35 PM
9/24/2019
hp
X](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb20bb9a2-6808-4be4-99b0-8e5526c57207%2F66155898-bf92-4c2e-84a8-7880100bd0ea%2Fkhjmqgb.jpeg&w=3840&q=75)
Transcribed Image Text:575718snapshotld=1324548& id-563649007&
Q Search this course
Reference
Use the References to access important va
eeded for this question.
The equilibrium constant, K, for the following reaction is 1.31x102 at 502 K.
PCI5(g) PCl3(g)+ C2(g)
An equilibrium mixture of the three gases in a 18.3 L container at 502 K contains 0.161 M
PCls, 4.59x10 M PCI3 and 4.59x102 M Cl,. What will be the concentrations of the three
gases once equilibrium has been reestablished, if the equilibrium mixture is compressed at
constant temperature to a volume of 7.43 L?
[PCI5]=
[PCI3]=
[Cl2]
Disturbing Equilibrium Volumes (Quantitative): This is group attempt 1 of 5
Next
Back
1:35 PM
9/24/2019
hp
X
Expert Solution
Step 1
Given the reaction is:
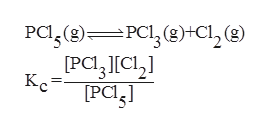
Step 2
After reestablishing equilibrium:
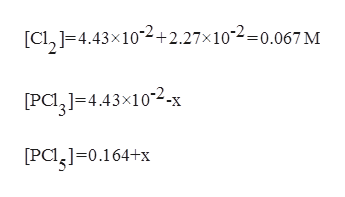
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning