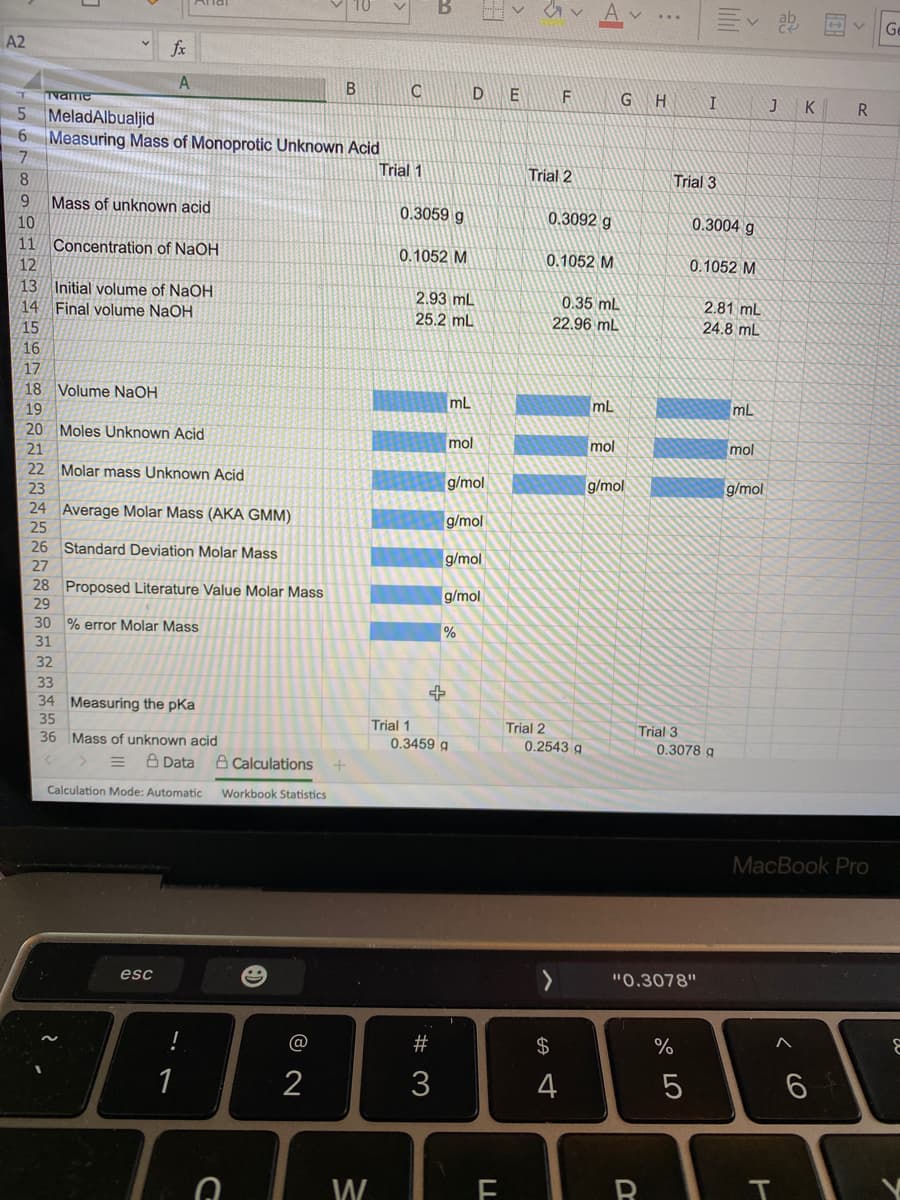
6 Measuring Mass of Monoprotic Unknown Acid Trial 1 Trial 2 Trial 3 8 Mass of unknown acid 10 0.3059 g 0.3092 g 0.3004 g 11 Concentration of NaOH 0.1052 M 0.1052 M 0.1052 M 12 13 Initial volume of NaOH 14 Final volume NaOH 2.93 mL 25.2 mL 0.35 mL 2.81 mL 22.96 mL 24.8 mL 15 16 17 18 Volume NAOH mL mL mL 19 20 Moles Unknown Acid mol mol mol 21 22 Molar mass Unknown Acid g/mol g/mol g/mol 23 24 Average Molar Mass (AKA GMM) 25 g/mol 26 Standard Deviation Molar Mass g/mol 27 28 Proposed Literature Value Molar Mass g/mol 29 30 % error Molar Mass 31 32
6 Measuring Mass of Monoprotic Unknown Acid Trial 1 Trial 2 Trial 3 8 Mass of unknown acid 10 0.3059 g 0.3092 g 0.3004 g 11 Concentration of NaOH 0.1052 M 0.1052 M 0.1052 M 12 13 Initial volume of NaOH 14 Final volume NaOH 2.93 mL 25.2 mL 0.35 mL 2.81 mL 22.96 mL 24.8 mL 15 16 17 18 Volume NAOH mL mL mL 19 20 Moles Unknown Acid mol mol mol 21 22 Molar mass Unknown Acid g/mol g/mol g/mol 23 24 Average Molar Mass (AKA GMM) 25 g/mol 26 Standard Deviation Molar Mass g/mol 27 28 Proposed Literature Value Molar Mass g/mol 29 30 % error Molar Mass 31 32
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.66PAE
Related questions
Question
100%
Please show calculations and fill in the blue boxes. Thank you
Transcribed Image Text:Ge
A2
fx
A
TName
H
I
K
R
5 MeladAlbualjid
Measuring Mass of Monoprotic Unknown Acid
7
Trial 1
Trial 2
Trial 3
8
Mass of unknown acid
10
0.3059 g
0.3092 g
0.3004 g
11 Concentration of NaOH
0.1052 M
0.1052 M
0.1052 M
12
13 Initial volume of NaOH
2.93 mL
25.2 mL
0.35 mL
22.96 mL
2.81 mL
14 Final volume NaOH
15
24.8 mL
16
17
18
Volume NaOH
mL
mL
mL
19
20 Moles Unknown Acid
mol
mol
mol
21
22 Molar mass Unknown Acid
|g/mol
g/mol
g/mol
23
24 Average Molar Mass (AKA GMM)
g/mol
25
26 Standard Deviation Molar Mass
g/mol
27
28 Proposed Literature Value Molar Mass
g/mol
29
30 % error Molar Mass
%
31
32
33
34 Measuring the pKa
35
Trial 1
0.3459 g
Trial 2
Trial 3
36 Mass of unknown acid
0.2543 g
0.3078 g
A Data
A Calculations
Calculation Mode: Automatic
Workbook Statistics
MacBook Pro
esc
"0.3078"
#3
2$
2
3
4
W
lilil
< CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning