6. Complete the following table: SOLUTE NaCl Urea CaCl₂ CaCl₂ MOLARITY (mole/L) 3 0.2 OSMOLARITY (osmol/L) 4 0.3 EXPLANATION TONICITY represents the ability of a solution to change the shape or turgidity of cells by altering their internal water volume (= the ability of a solution to grab water from the cells). Every cell, including the red-blood cell, contains within the boundary of its cell membrane a certain number of non-penetrating solute particles (0.3 osmol/L) which will not diffuse out of th cell.
6. Complete the following table: SOLUTE NaCl Urea CaCl₂ CaCl₂ MOLARITY (mole/L) 3 0.2 OSMOLARITY (osmol/L) 4 0.3 EXPLANATION TONICITY represents the ability of a solution to change the shape or turgidity of cells by altering their internal water volume (= the ability of a solution to grab water from the cells). Every cell, including the red-blood cell, contains within the boundary of its cell membrane a certain number of non-penetrating solute particles (0.3 osmol/L) which will not diffuse out of th cell.
Biomedical Instrumentation Systems
1st Edition
ISBN:9781133478294
Author:Chatterjee
Publisher:Chatterjee
Chapter9: Instrumentation In Blood Circulation
Section: Chapter Questions
Problem 13P
Related questions
Question
please answer 6
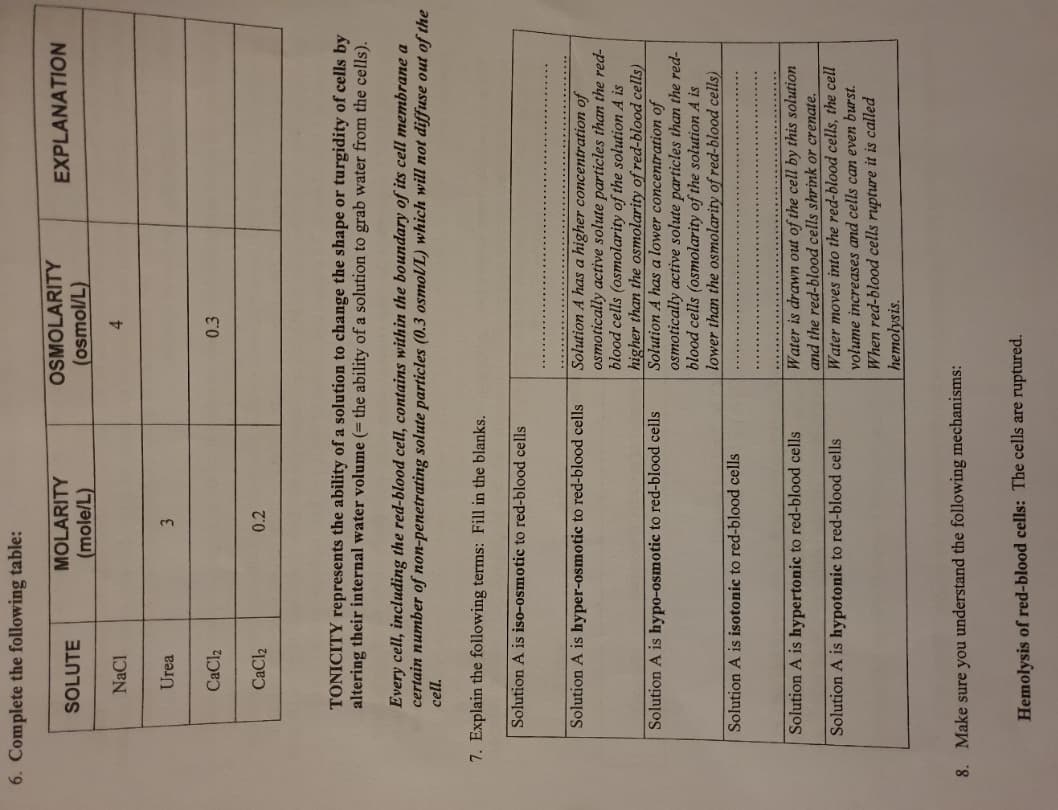
Transcribed Image Text:6. Complete the following table:
SOLUTE
NaCl
Urea
CaCl₂
CaCl₂
MOLARITY
(mole/L)
3
0.2
7. Explain the following terms: Fill in the blanks.
Solution A is iso-osmotic to red-blood cells
TONICITY represents the ability of a solution to change the shape or turgidity of cells by
altering their internal water volume (= the ability of a solution to grab water from the cells).
Solution A is hyper-osmotic to red-blood cells
Every cell, including the red-blood cell, contains within the boundary of its cell membrane a
certain number of non-penetrating solute particles (0.3 osmol/L) which will not diffuse out of the
cell.
Solution A is hypo-osmotic to red-blood cells
OSMOLARITY
(osmol/L)
4
Solution A is isotonic to red-blood cells
Solution A is hypertonic to red-blood cells
Solution A is hypotonic to red-blood cells
0.3
EXPLANATION
Solution A has a higher concentration of
osmotically active solute particles than the red-
blood cells (osmolarity of the solution A is
higher than the osmolarity of red-blood cells)
Solution A has a lower concentration of
osmotically active solute particles than the red-
blood cells (osmolarity of the solution A is
lower than the osmolarity of red-blood cells)
Water is drawn out of the cell by this solution
and the red-blood cells shrink or crenate.
Water moves into the red-blood cells, the cell
volume increases and cells can even burst.
1-blood cells rupture it is called
hemolysis.
8. Make sure you understand the following mechanisms:
Hemolysis of red-blood cells: The cells are ruptured.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you



