6. Determine the ionization energy for the hydrogen atom. The ionization energy is the energy necessary to take an electron from its most stable state (n, = 1 for hydrogen), to a state infi- nitely far from the nucleus. Use Equation 3 to make the calculation. What is n? Show your work. he hckH Equation 3: A E 2. n
6. Determine the ionization energy for the hydrogen atom. The ionization energy is the energy necessary to take an electron from its most stable state (n, = 1 for hydrogen), to a state infi- nitely far from the nucleus. Use Equation 3 to make the calculation. What is n? Show your work. he hckH Equation 3: A E 2. n
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 109E
Related questions
Question
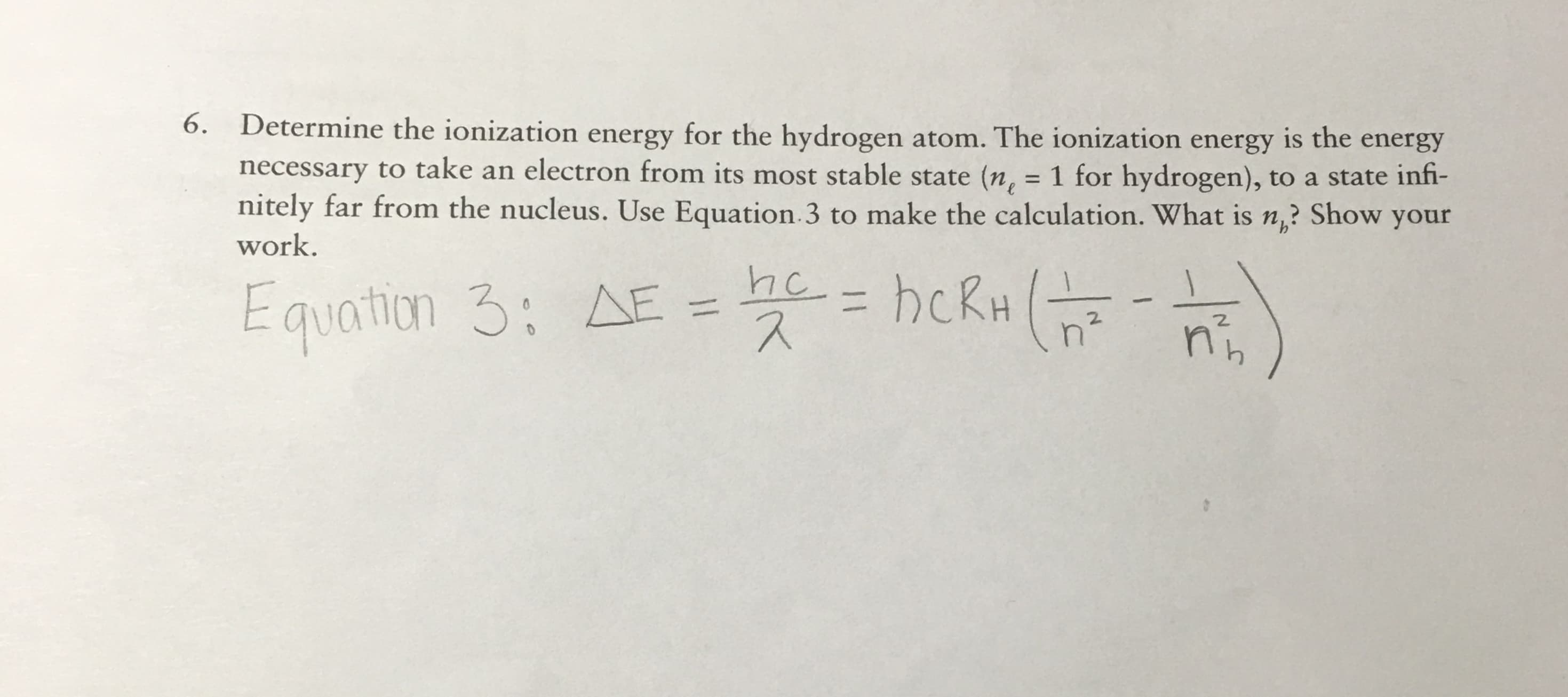
Transcribed Image Text:6. Determine the ionization energy for the hydrogen atom. The ionization energy is the energy
necessary to take an electron from its most stable state (n, = 1 for hydrogen), to a state infi-
nitely far from the nucleus. Use Equation 3 to make the calculation. What is n? Show your
work.
he hckH
Equation 3: A
E
2.
n
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
