61% iPad 2:20 PM + openvellum.ecollege.com Search Textbook Solutions | Chegg.com Answered: Which of the following statements about... Course Home Molecules of Life Help Sign Out Hi, Ashly Mastering Chemistry Course Home My Courses KOnline homework for CHO8 Course Home Problem 8.42 33 of 41 Syllabus Scores Review | Constants I Periodic Table еТext Part A Document Sharing How would you prepare 2 L of 1 M MgCl2 from a 4 M MĚCL2 stock solution? User Settings Add 500 mL of the 4 M MgCl, stock solution to enough distilled water for a total volume of 2 L of solution. Course Tools Add 2000 mlL of the 4 M MgCl2 stock solution to enough distilled water for a total volume of 2 Lof solution. Add 250 mL of the 4 M MgCl2 stock solution to enough distilled water for a total volume of 2 L of solution. Request Answer Submit Provide Feedback Next
61% iPad 2:20 PM + openvellum.ecollege.com Search Textbook Solutions | Chegg.com Answered: Which of the following statements about... Course Home Molecules of Life Help Sign Out Hi, Ashly Mastering Chemistry Course Home My Courses KOnline homework for CHO8 Course Home Problem 8.42 33 of 41 Syllabus Scores Review | Constants I Periodic Table еТext Part A Document Sharing How would you prepare 2 L of 1 M MgCl2 from a 4 M MĚCL2 stock solution? User Settings Add 500 mL of the 4 M MgCl, stock solution to enough distilled water for a total volume of 2 L of solution. Course Tools Add 2000 mlL of the 4 M MgCl2 stock solution to enough distilled water for a total volume of 2 Lof solution. Add 250 mL of the 4 M MgCl2 stock solution to enough distilled water for a total volume of 2 L of solution. Request Answer Submit Provide Feedback Next
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 66PS
Related questions
Question
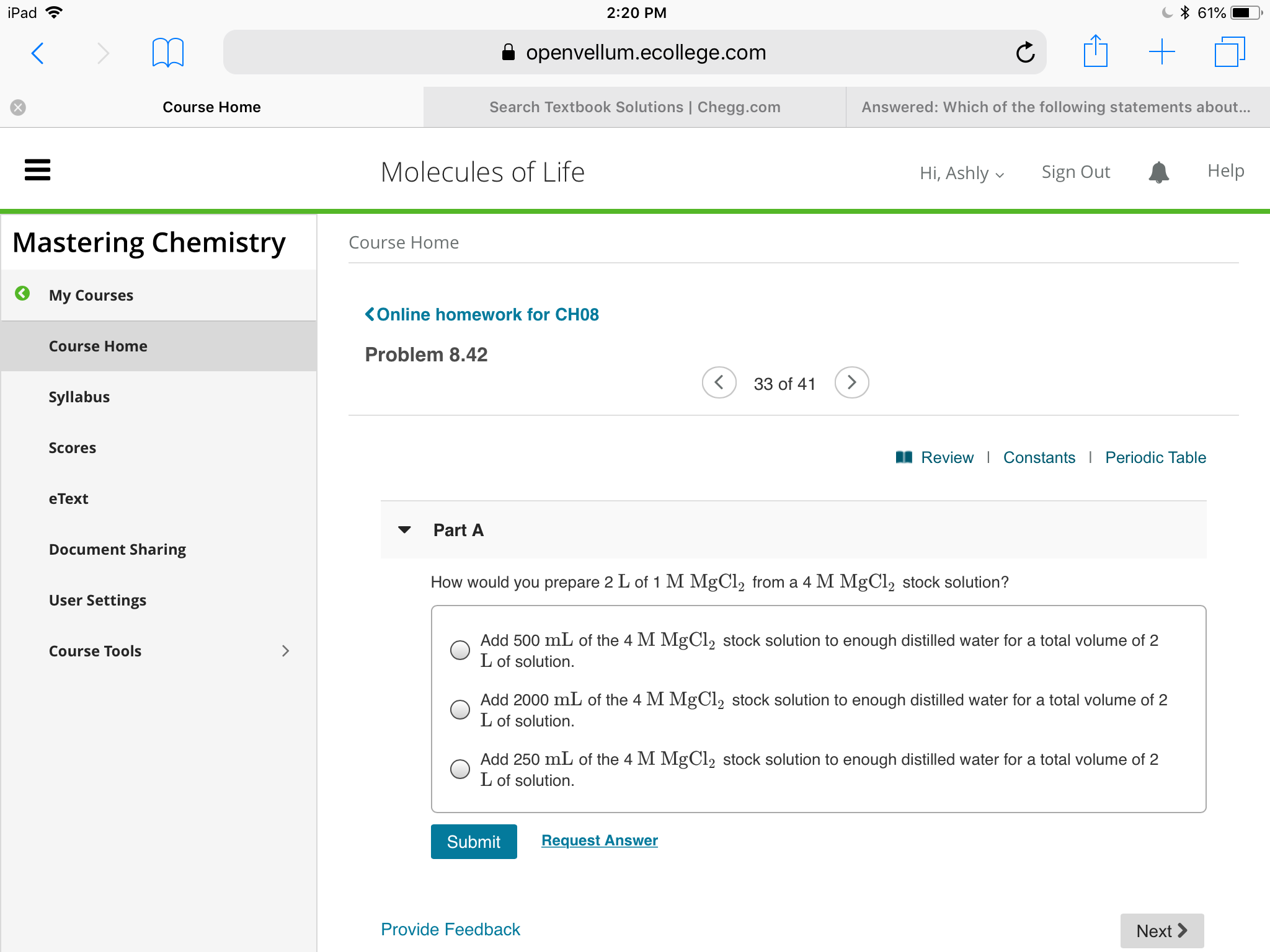
Transcribed Image Text:61%
iPad
2:20 PM
+
openvellum.ecollege.com
Search Textbook Solutions | Chegg.com
Answered: Which of the following statements about...
Course Home
Molecules of Life
Help
Sign Out
Hi, Ashly
Mastering Chemistry
Course Home
My Courses
KOnline homework for CHO8
Course Home
Problem 8.42
33 of 41
Syllabus
Scores
Review | Constants I Periodic Table
еТext
Part A
Document Sharing
How would you prepare 2 L of 1 M MgCl2 from a 4 M MĚCL2 stock solution?
User Settings
Add 500 mL of the 4 M MgCl, stock solution to enough distilled water for a total volume of 2
L of solution.
Course Tools
Add 2000 mlL of the 4 M MgCl2 stock solution to enough distilled water for a total volume of 2
Lof solution.
Add 250 mL of the 4 M MgCl2 stock solution to enough distilled water for a total volume of 2
L of solution.
Request Answer
Submit
Provide Feedback
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning