68 The Organic Chemistry Laboratory Experience 4. What would be the expected product for each of the following reactions? Y OMe 8YXOHT3IMIC AICI3 -CI 2920190 toda I-g excess amak bonld rol (milm OEt o woms bavnus он H+ excess
68 The Organic Chemistry Laboratory Experience 4. What would be the expected product for each of the following reactions? Y OMe 8YXOHT3IMIC AICI3 -CI 2920190 toda I-g excess amak bonld rol (milm OEt o woms bavnus он H+ excess
Chapter25: Biomolecules: Carbohydrates
Section25.SE: Something Extra
Problem 49AP
Related questions
Question
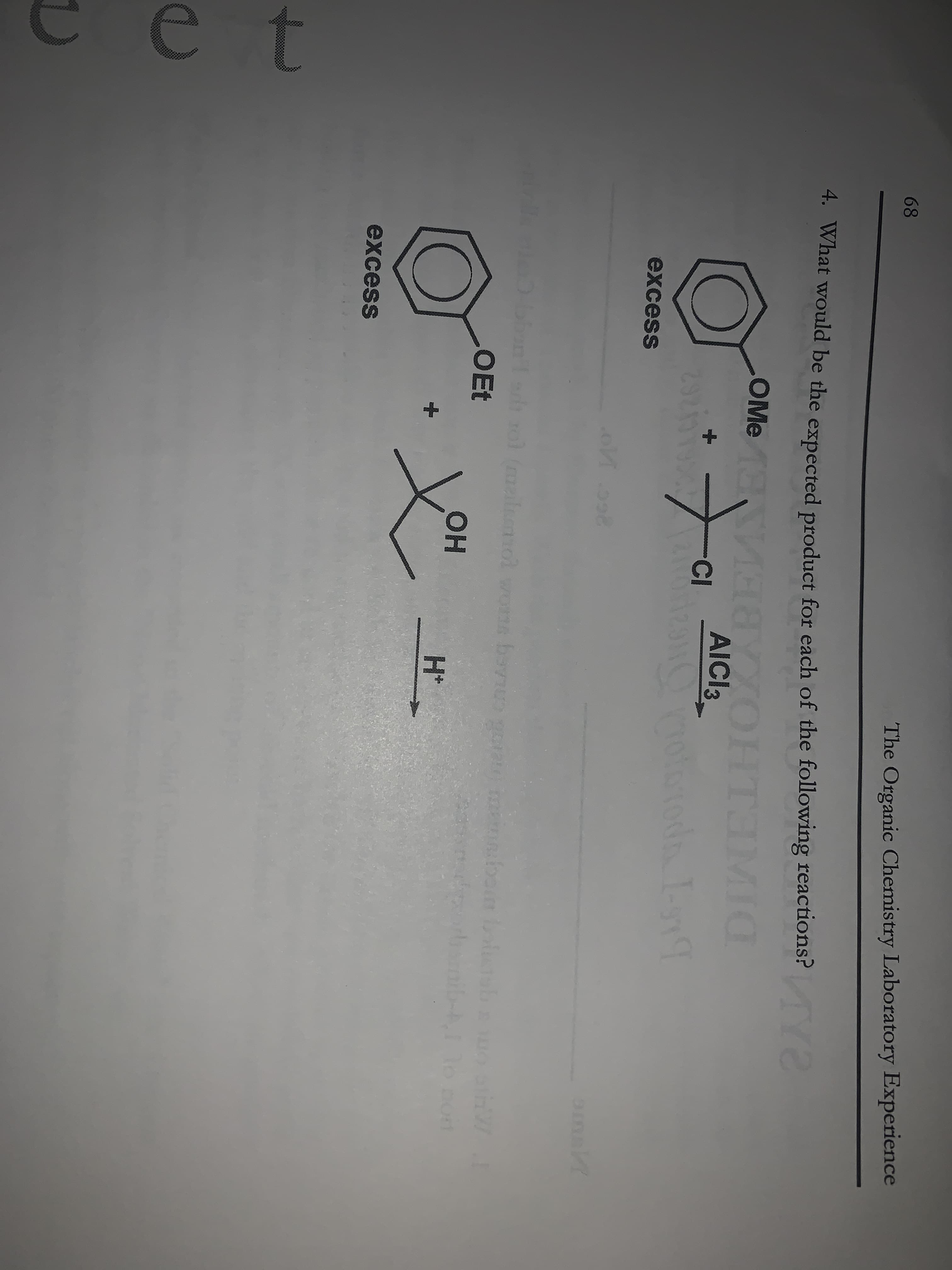
Transcribed Image Text:68
The Organic Chemistry Laboratory Experience
4. What would be the expected product for each of the following reactions?
Y
OMe
8YXOHT3IMIC
AICI3
-CI
2920190
toda I-g
excess
amak
bonld
rol (milm
OEt
o woms bavnus
он
H+
excess
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning