Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 10CTQ
Related questions
Question
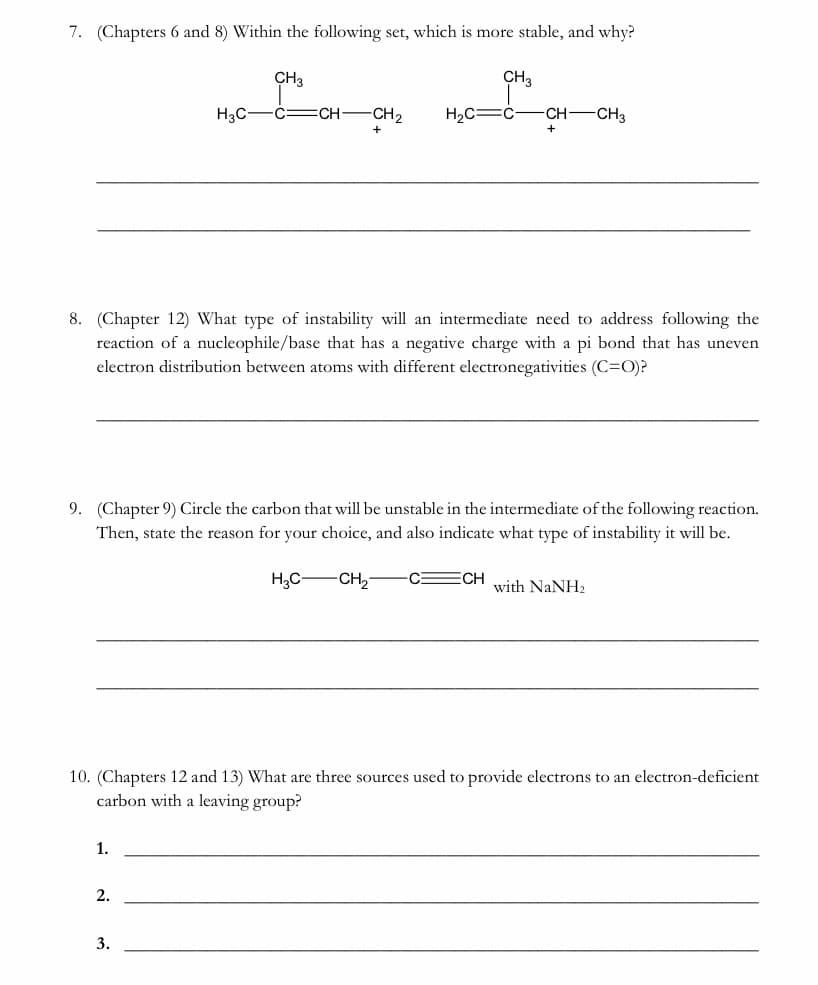
Transcribed Image Text:7. (Chapters 6 and 8) Within the following set, which is more stable, and why?
CH3
CH3
H3C-
-C=CH-
CH2
H2C=Ć-
-CH CH3
8. (Chapter 12) What type of instability will an intermediate need to address following the
reaction of a nucleophile/base that has a negative charge with a pi bond that has uneven
electron distribution between atoms with different electronegativities (C=O)?
9. (Chapter 9) Circle the carbon that will be unstable in the intermediate of the following reaction.
Then, state the reason for your choice, and also indicate what type of instability it will be.
H,C-CH,-
C ECH
with NaNH2
10. (Chapters 12 and 13) What are three sources used to provide electrons to an electron-deficient
carbon with a leaving group?
1.
2.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
