Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:7:40
session.masteringchemistry.com/myct/itemView?offset=next&attemptNo=1&ass
:D
<Chapter 15 - Attempt 1
+ Pressure-Based versus Concentration-Based Equilibrium Constants
3 of 11
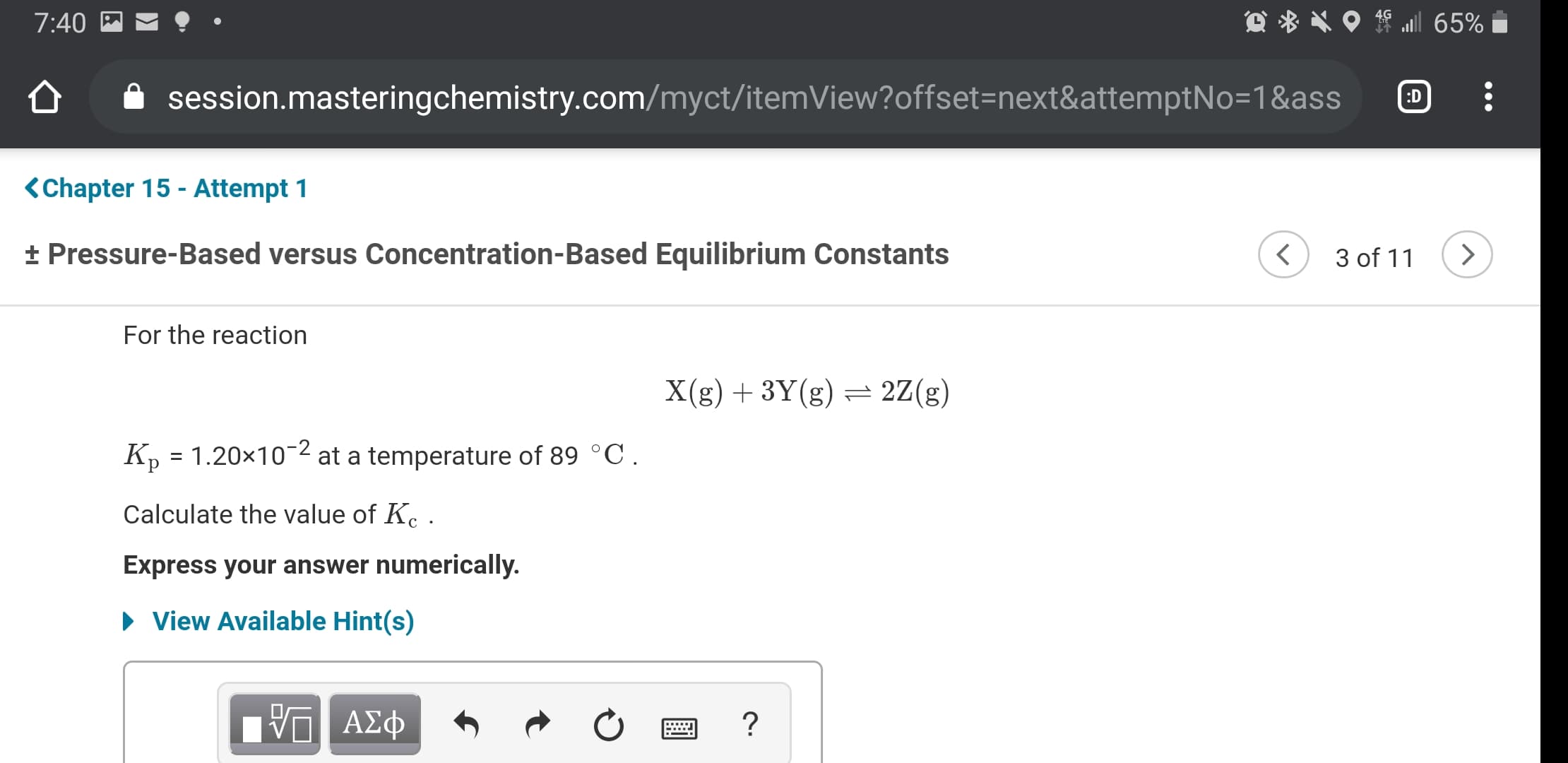
For the reaction
X(g) + 3Y(g) = 2Z(g)
K, = 1.20x10-2 at a temperature of 89 °C.
Calculate the value of K. .
Express your answer numerically.
• View Available Hint(s)
ΑΣφ
![7:41
session.masteringchemistry.com/myct/itemView?assignmentProblemID=141619
:D
<Chapter 15 - Attempt 1
Exercise 15.36 - Enhanced - with Feedback
4 of 11
Review
COnstanIS
PenodioC Tabie
Consider the following reaction:
NH,HS (s) = NH3 (g) + H2S (g)
An equilibrium mixture of this reaction at a certain temperature was found to have NH3]
0.282 Mand [H2S] =
0.370 M.
You may want to reference (Pages 651 - 653) Section 15.6 while completing this problem.
Part A](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd505aa60-a219-4ab9-8e2f-c229b462f4c9%2F60394381-cc80-40c3-b7a1-07d6db36c8ec%2F1pchbzl.jpeg&w=3840&q=75)
Transcribed Image Text:7:41
session.masteringchemistry.com/myct/itemView?assignmentProblemID=141619
:D
<Chapter 15 - Attempt 1
Exercise 15.36 - Enhanced - with Feedback
4 of 11
Review
COnstanIS
PenodioC Tabie
Consider the following reaction:
NH,HS (s) = NH3 (g) + H2S (g)
An equilibrium mixture of this reaction at a certain temperature was found to have NH3]
0.282 Mand [H2S] =
0.370 M.
You may want to reference (Pages 651 - 653) Section 15.6 while completing this problem.
Part A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY