813056575718snapshotld=13245488id-563649068&takeld=4d7290492 b0cf5b& Q Search Scoring: Your score will be based on the number of correct matches minus the number of incorrect matches. There is no penalty for missing matches. References Use the References to access important values if needed for this question. For the following reaction, K< 1. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases. C5H5N+ HC,H-O4 CoH,O4 CsHzNH Clear All Stronger Bronsted-Lowry acid CoН,04 1. Classify B-L Acids/Bases by Strength: This is group attempt 1 of 5 Regenerate Grou Autosaved at 10:45 PM Next Back 10:4 е 10/14 Stronger Bronsted-Lowry CoH-O4 acid Weaker Bronsted-Lowry CgH5NH acid Stronger Bronsted-Lowry C5H5N base Weaker Bronsted-Lowry HC,H,O4 base fy B-L Acids/Bases by Strength: This is group attempt 1 of 5 Autosaved at 10:45 PM thp
813056575718snapshotld=13245488id-563649068&takeld=4d7290492 b0cf5b& Q Search Scoring: Your score will be based on the number of correct matches minus the number of incorrect matches. There is no penalty for missing matches. References Use the References to access important values if needed for this question. For the following reaction, K< 1. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases. C5H5N+ HC,H-O4 CoH,O4 CsHzNH Clear All Stronger Bronsted-Lowry acid CoН,04 1. Classify B-L Acids/Bases by Strength: This is group attempt 1 of 5 Regenerate Grou Autosaved at 10:45 PM Next Back 10:4 е 10/14 Stronger Bronsted-Lowry CoH-O4 acid Weaker Bronsted-Lowry CgH5NH acid Stronger Bronsted-Lowry C5H5N base Weaker Bronsted-Lowry HC,H,O4 base fy B-L Acids/Bases by Strength: This is group attempt 1 of 5 Autosaved at 10:45 PM thp
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter18: More Statistical Thermodynamics
Section: Chapter Questions
Problem 18.51E
Related questions
Question
The following reactions all have K > 1.
1) C9H7O4- (aq) + HF (aq) HC9H7O4 (aq) + F- (aq)
2) CH3COO- (aq) + HF (aq) F- (aq) + CH3COOH (aq)
3) HC9H7O4 (aq) + CH3COO- (aq) CH3COOH (aq) + C9H7O4- (aq)
Arrange the substances based on their relative acid strength.
CH3COOH
HF
F-
C9H7O4-
HC9H7O4
CH3COO-
strongest acid
intermediate acid
weakest acid
not a Bronsted-Lowry acid
Transcribed Image Text:813056575718snapshotld=13245488id-563649068&takeld=4d7290492 b0cf5b&
Q Search
Scoring: Your score will be based on the number of correct matches minus the number of
incorrect matches. There is no penalty for missing matches.
References
Use the References to access important values if needed for this question.
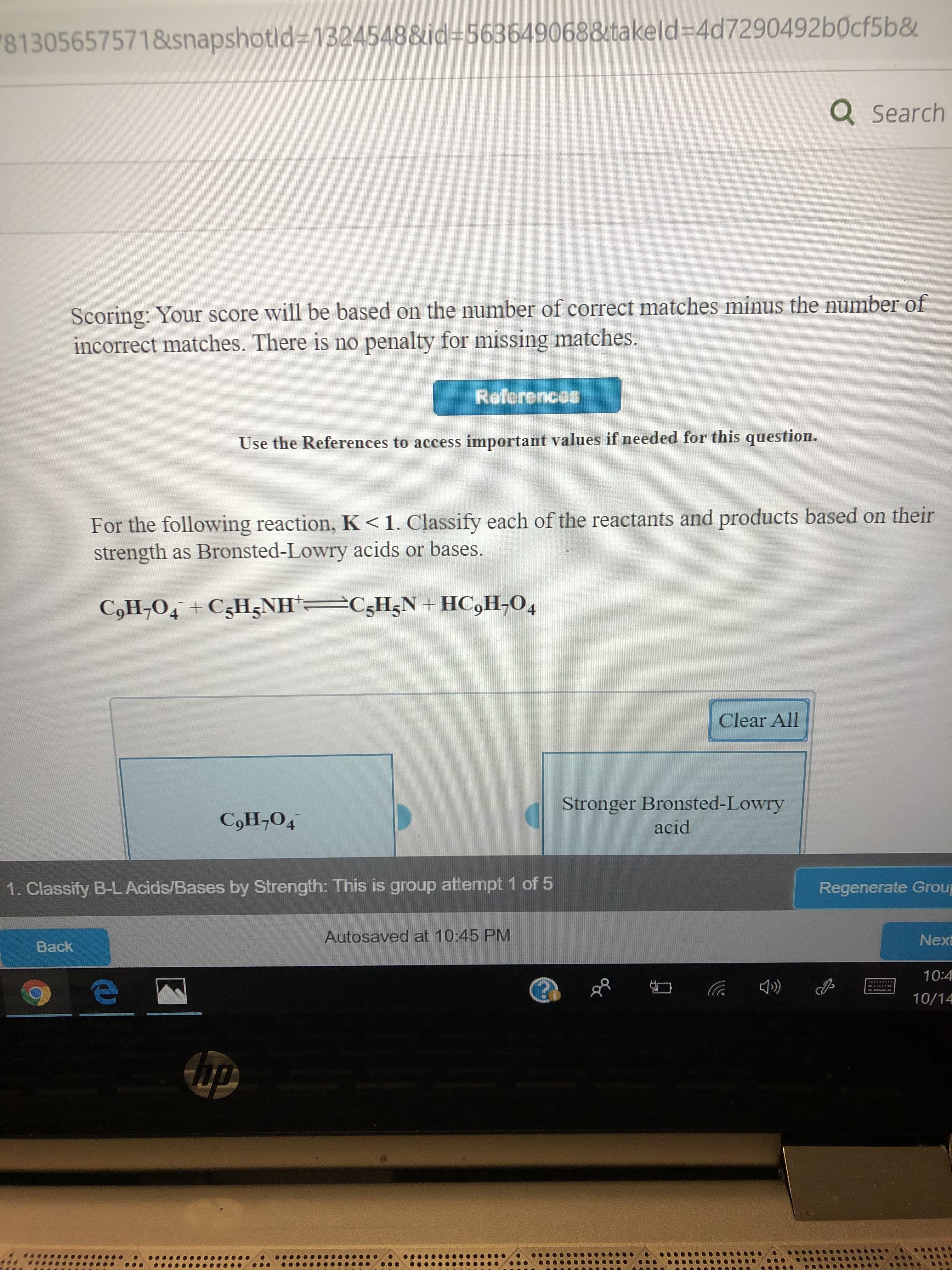
For the following reaction, K< 1. Classify each of the reactants and products based on their
strength as Bronsted-Lowry acids or bases.
C5H5N+ HC,H-O4
CoH,O4 CsHzNH
Clear All
Stronger Bronsted-Lowry
acid
CoН,04
1. Classify B-L Acids/Bases by Strength: This is group attempt 1 of 5
Regenerate Grou
Autosaved at 10:45 PM
Next
Back
10:4
е
10/14
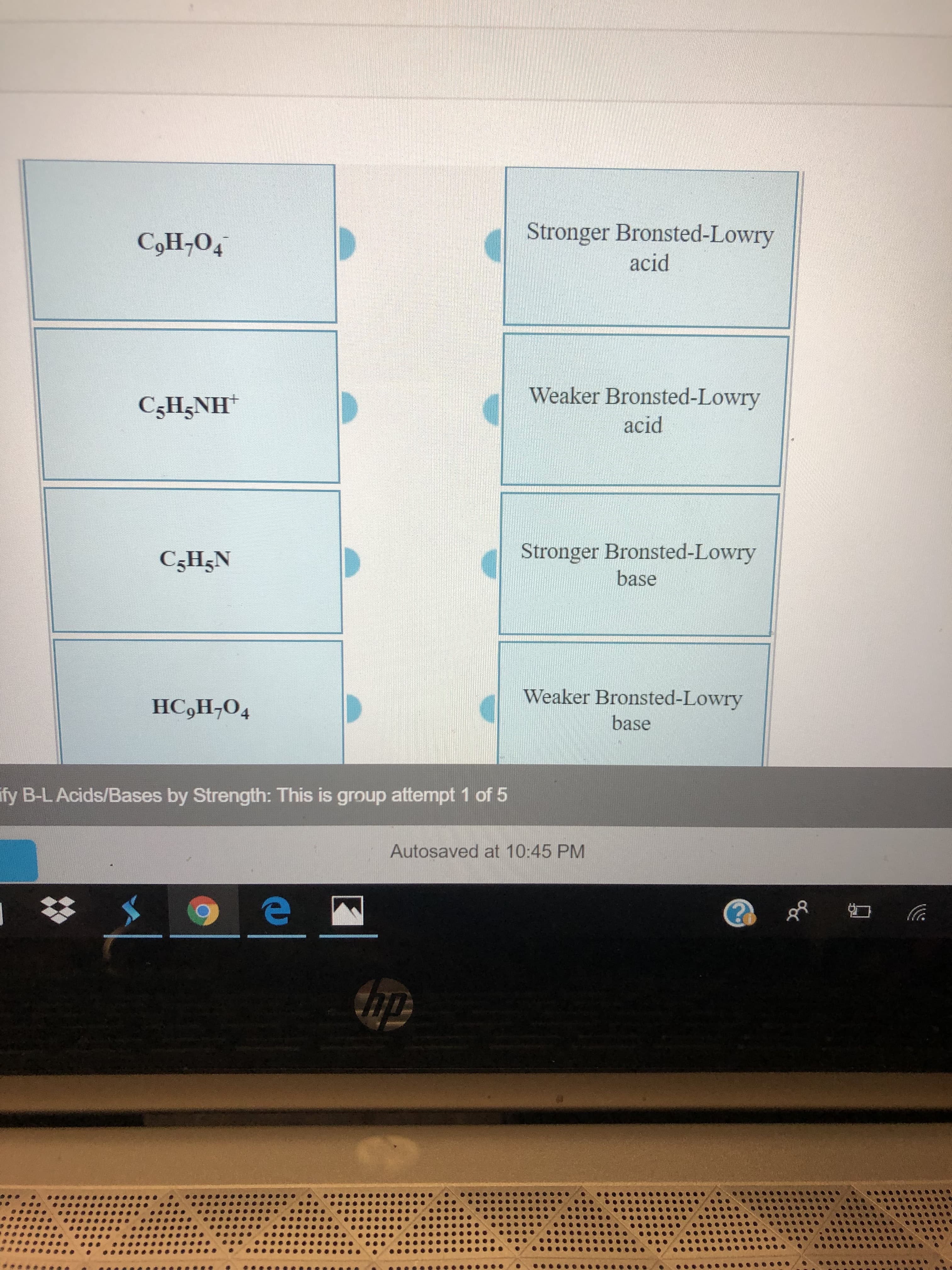
Transcribed Image Text:Stronger Bronsted-Lowry
CoH-O4
acid
Weaker Bronsted-Lowry
CgH5NH
acid
Stronger Bronsted-Lowry
C5H5N
base
Weaker Bronsted-Lowry
HC,H,O4
base
fy B-L Acids/Bases by Strength: This is group attempt 1 of 5
Autosaved at 10:45 PM
thp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning