9- NUTRIE x M Inbox (623 M Mr leahy G is enthalpy X Physical a X 9 АСE251_Dх ACE251 D X RLON-CAPA X BB Welcome, X Course Po X X access2.lon-capa.uiuc.edu/res/uiuc/cyerkes/Chem204/Homework_3/work.problem?symb=uploaded%2fuiuc%2f63206040cd3285d3euiuclibrary1%2fdefault_1414942219%2esequence - section: CQB) (Student CHEM 102B/C Fall 2019 Messages Courses Help Logout Lorcan James McGrath Main Menu Contents Grades Course Contents » ... » Homework 12 » Homework 12-4 Timer Calculate the work (w) and AE°, in kJ, at 298 K and 1 atm pressure, for the combustion of one mole of C4H10 (g). First write and balance the equation. The products will be CO2 (g) and H20 (g) The value of AH° for this reaction is -2658.3 kJ/mol. The value for w in kJ = Tries 0/99 Submit Answer The value for AE° ( in kJ) = Tries 0/99 Submit Answer Threaded View Chronological View Other Views... Export My general preferences on what is marked as NEW Mark NEW posts no longer new NEW Anonymous 1 Reply (Sun Nov 17 10:01:11 am 2019 (CST)) how do you find the change in volume so you can put it in the w=-PV equation NEW Re: Anonymous 2 Reply (Sun Nov 17 11:56:05 am 2019 (CST)) I think you use the number of moles in the reactants and products in two equations then compare the volumes Erin McKenzie Reply (Sun Nov 17 04:49:51 pm 2019 (CST)) NEW what do yo do to get the enthalpy fromkj/mol to just kj? NEW Re: Anonymous 1 Reply (Sun Nov 17 05:36:07 pm 2019 (CST)) the unit is in kj/mol so if you multiply by mol they cross out and u just get mol. all units do that in dimensional analysis My settings for this discussion: 1. Display - All posts 2. Not new - Once marked not NEW Change My_general preferences on what is marked as NEW Mark NEW posts no longer new Threaded View Export Chronological View Other Views.. (В е вы sky |Bb 21:44 sports ..
9- NUTRIE x M Inbox (623 M Mr leahy G is enthalpy X Physical a X 9 АСE251_Dх ACE251 D X RLON-CAPA X BB Welcome, X Course Po X X access2.lon-capa.uiuc.edu/res/uiuc/cyerkes/Chem204/Homework_3/work.problem?symb=uploaded%2fuiuc%2f63206040cd3285d3euiuclibrary1%2fdefault_1414942219%2esequence - section: CQB) (Student CHEM 102B/C Fall 2019 Messages Courses Help Logout Lorcan James McGrath Main Menu Contents Grades Course Contents » ... » Homework 12 » Homework 12-4 Timer Calculate the work (w) and AE°, in kJ, at 298 K and 1 atm pressure, for the combustion of one mole of C4H10 (g). First write and balance the equation. The products will be CO2 (g) and H20 (g) The value of AH° for this reaction is -2658.3 kJ/mol. The value for w in kJ = Tries 0/99 Submit Answer The value for AE° ( in kJ) = Tries 0/99 Submit Answer Threaded View Chronological View Other Views... Export My general preferences on what is marked as NEW Mark NEW posts no longer new NEW Anonymous 1 Reply (Sun Nov 17 10:01:11 am 2019 (CST)) how do you find the change in volume so you can put it in the w=-PV equation NEW Re: Anonymous 2 Reply (Sun Nov 17 11:56:05 am 2019 (CST)) I think you use the number of moles in the reactants and products in two equations then compare the volumes Erin McKenzie Reply (Sun Nov 17 04:49:51 pm 2019 (CST)) NEW what do yo do to get the enthalpy fromkj/mol to just kj? NEW Re: Anonymous 1 Reply (Sun Nov 17 05:36:07 pm 2019 (CST)) the unit is in kj/mol so if you multiply by mol they cross out and u just get mol. all units do that in dimensional analysis My settings for this discussion: 1. Display - All posts 2. Not new - Once marked not NEW Change My_general preferences on what is marked as NEW Mark NEW posts no longer new Threaded View Export Chronological View Other Views.. (В е вы sky |Bb 21:44 sports ..
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.32E: Many compressed gases come in large,heavy metal cylindersthat are so heavy that they need a special...
Related questions
Question
Transcribed Image Text:9- NUTRIE x M Inbox (623 M Mr leahy
G is enthalpy X
Physical a X
9 АСE251_Dх
ACE251 D X
RLON-CAPA X
BB Welcome, X
Course Po X
X
access2.lon-capa.uiuc.edu/res/uiuc/cyerkes/Chem204/Homework_3/work.problem?symb=uploaded%2fuiuc%2f63206040cd3285d3euiuclibrary1%2fdefault_1414942219%2esequence
- section: CQB)
(Student
CHEM 102B/C Fall 2019
Messages Courses Help Logout
Lorcan James McGrath
Main Menu
Contents
Grades
Course Contents » ... » Homework 12 » Homework 12-4
Timer
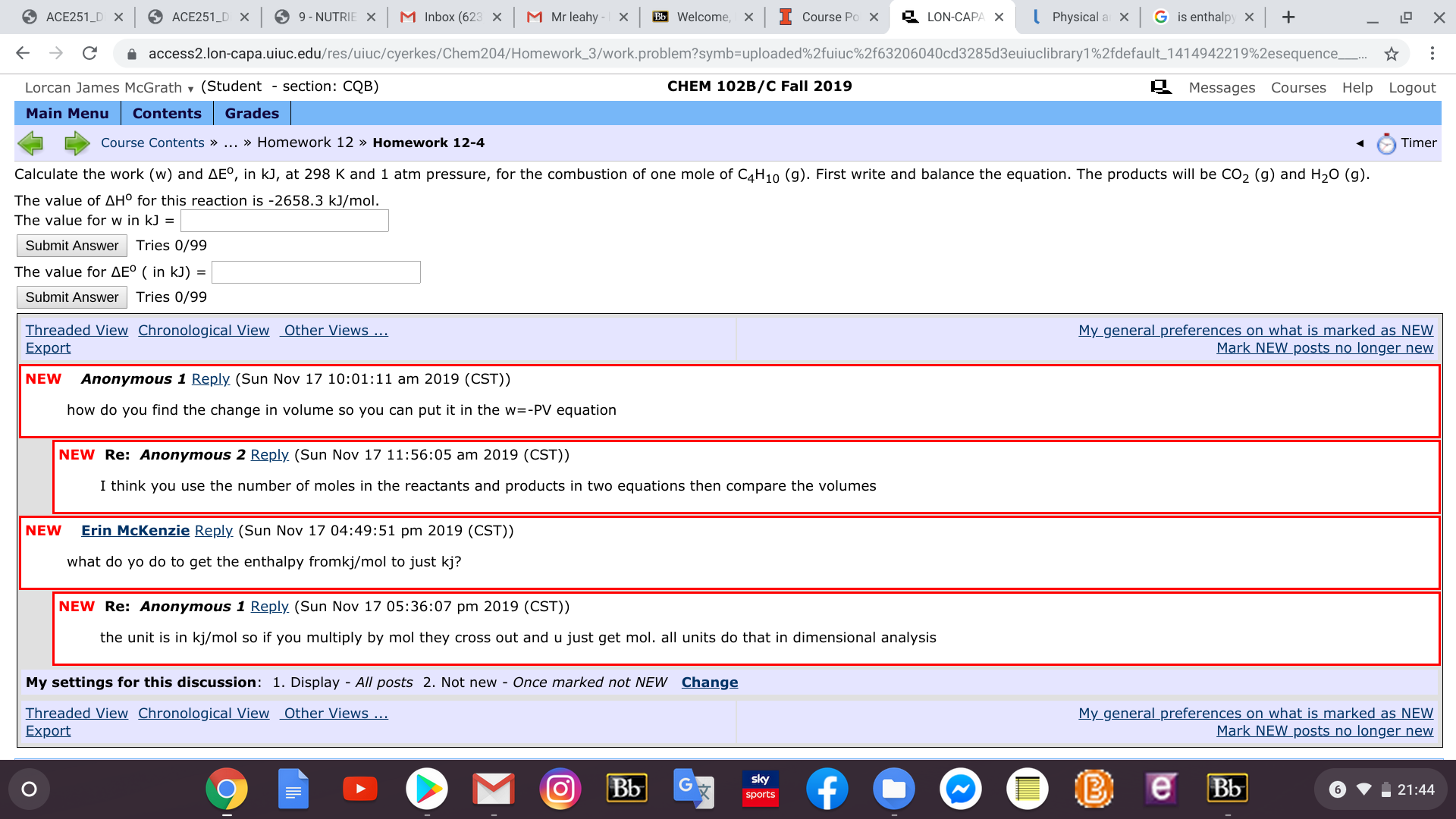
Calculate the work (w) and AE°, in kJ, at 298 K and 1 atm pressure, for the combustion of one mole of C4H10 (g). First write and balance the equation. The products will be CO2 (g) and H20 (g)
The value of AH° for this reaction is -2658.3 kJ/mol.
The value for w in kJ =
Tries 0/99
Submit Answer
The value for AE° ( in kJ) =
Tries 0/99
Submit Answer
Threaded View Chronological View Other Views...
Export
My general preferences on what is marked as NEW
Mark NEW posts no longer new
NEW
Anonymous 1 Reply (Sun Nov 17 10:01:11 am 2019 (CST))
how do you find the change in volume so you can put it in the w=-PV equation
NEW Re: Anonymous 2 Reply (Sun Nov 17 11:56:05 am 2019 (CST))
I think you use the number of moles in the reactants and products in two equations then compare the volumes
Erin McKenzie Reply (Sun Nov 17 04:49:51 pm 2019 (CST))
NEW
what do yo do to get the enthalpy fromkj/mol to just kj?
NEW Re: Anonymous 1 Reply (Sun Nov 17 05:36:07 pm 2019 (CST))
the unit is in kj/mol so if you multiply by mol they cross out and u just get mol. all units do that in dimensional analysis
My settings for this discussion: 1. Display - All posts 2. Not new - Once marked not NEW Change
My_general preferences on what is marked as NEW
Mark NEW posts no longer new
Threaded View
Export
Chronological View
Other Views..
(В е вы
sky
|Bb
21:44
sports
..
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,