9) Remove the metal wires from their solutions. Rinse them with water and dry them with a paper towel. Retain the copper wire and replace the zinc wire in the container in the hood. In-Lab Discussion 1. When zinc metal reacts with Cu2+ ions, which substance is oxidized and which is reduced? 2. Which metal is the anode in this electrochemical cell? Which is the cathode? 3. In which direction do the electrons flow in this cell? From the zinc wire to the copper metal wire, or vice versa? 4. What would you conclude about the spontaneity of the reaction if you replaced the zinc wire with another metal and found that the cell potential changed sign?
9) Remove the metal wires from their solutions. Rinse them with water and dry them with a paper towel. Retain the copper wire and replace the zinc wire in the container in the hood. In-Lab Discussion 1. When zinc metal reacts with Cu2+ ions, which substance is oxidized and which is reduced? 2. Which metal is the anode in this electrochemical cell? Which is the cathode? 3. In which direction do the electrons flow in this cell? From the zinc wire to the copper metal wire, or vice versa? 4. What would you conclude about the spontaneity of the reaction if you replaced the zinc wire with another metal and found that the cell potential changed sign?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.12QAP: What arc the advantages of microfabricated ISEs? Describe typical applications of this type of...
Related questions
Question
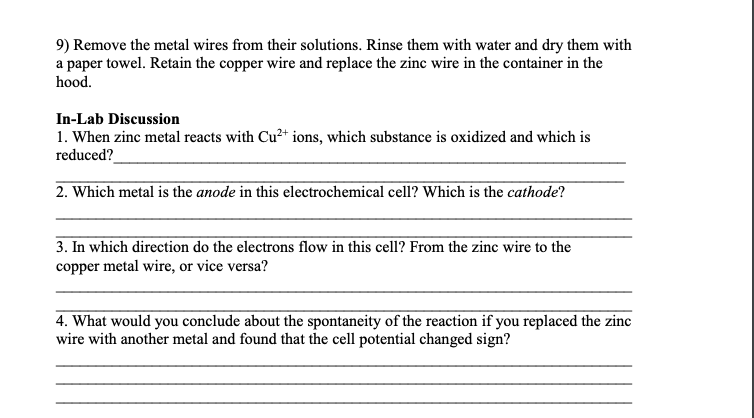
Transcribed Image Text:9) Remove the metal wires from their solutions. Rinse them with water and dry them with
a paper towel. Retain the copper wire and replace the zinc wire in the container in the
hood.
In-Lab Discussion
1. When zinc metal reacts with Cu2+ ions, which substance is oxidized and which is
reduced?
2. Which metal is the anode in this electrochemical cell? Which is the cathode?
3. In which direction do the electrons flow in this cell? From the zinc wire to the
copper metal wire, or vice versa?
4. What would you conclude about the spontaneity of the reaction if you replaced the zinc
wire with another metal and found that the cell potential changed sign?

Transcribed Image Text:Filter paper
Saturated KNO; solution
259
Figure 1. Experimental apparatus.
Experimental Procedure
Part 1. Copper and Zinc Metal
1) Select a piece of copper and a piece of zinc metal wire from the containers in the hood.
2) Clean the surface of the metal wires with the steel wool. Wipe clean with a dry paper
towel.
3) Obtain a plastic well plate. Fill one well with 10 drops of the Copper ion solution from
the dropper bottle. Label this well with a Sharpie marker so that you remember which
solution you filled it with,
4) Repeat step 3 with Zinc ion solution. Select a well right next to the well in step 3 so
that the two wells with Copper and Zinc ion solution are right next to each other.
5) Obtain a small dry strip of filter paper. You may have to cut a large piece of filter
paper down to the appropriate size. It should be exactly this big C
It's OK to tear the filter paper with your hands. Using the drop bottle, saturate the filter
paper strip with KNO; solution. Gently place one end of the filter paper into one of the
ion solution wells and the other end of the filter paper into the second ion solution well so
that a bridge is formed between the two solutions.
6) Plug the alligator clips in to the voltmeter. The red lead connects to the voltmeter at the
V Q Hz connection. The black lead connects to the COM connection. Connect the red
alligator clip to one of the metal wires you chose and the black alligator clip to the other
metal wire you chose (make sure you clip on the metal and not the painted portion of the
wire). Turn the voltmeter knob to 2 _V and power on by pressing .
7) Dip both metal wires into their corresponding ion solutions and hold them there. If the
reading from the voltmeter is negative, switch the alligator clips for the two metal wires
so that the potential is positive.
8) Record the magnitude of the potential difference in the table provided.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning