9:46 6. ... Unanswered • 1 attempt left • Due on Oct 20, 7:00 PM How much heat is involved when 68.0 g of N2 are reacted in the reaction: 2N2(g) + 502(g) + 2H2O(1) → 4HNO3(aq) AH° = -256 kJ A -134 kJ -311 kJ C -356 kJ D -671 kJ E -622 kJ A Submit
9:46 6. ... Unanswered • 1 attempt left • Due on Oct 20, 7:00 PM How much heat is involved when 68.0 g of N2 are reacted in the reaction: 2N2(g) + 502(g) + 2H2O(1) → 4HNO3(aq) AH° = -256 kJ A -134 kJ -311 kJ C -356 kJ D -671 kJ E -622 kJ A Submit
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 75E: The white pigment TiO2 is prepared by the reaction of titanium tetrachioride, TiCl4, with water...
Related questions
Question
Transcribed Image Text:9:46
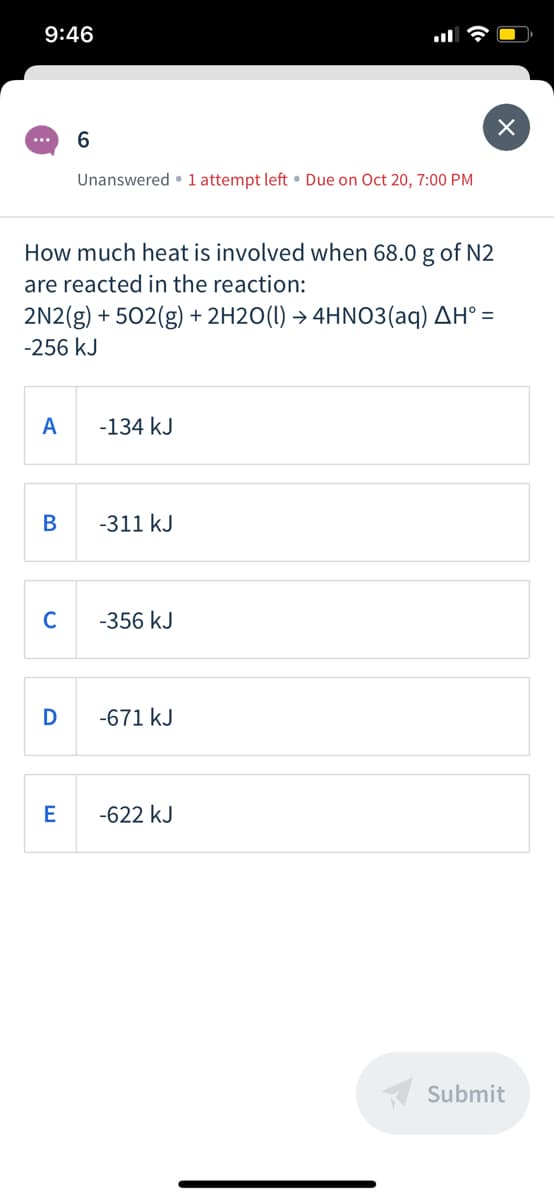
6.
...
Unanswered • 1 attempt left • Due on Oct 20, 7:00 PM
How much heat is involved when 68.0 g of N2
are reacted in the reaction:
2N2(g) + 502(g) + 2H2O(1) → 4HNO3(aq) AH° =
-256 kJ
A
-134 kJ
-311 kJ
C
-356 kJ
D
-671 kJ
E
-622 kJ
A Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning