* 97% 7:57 PM Wed Feb 12 х Unanswered •1 attempt left • Due on Feb 13 The initial concentration of reactant of a 2nd order reaction is 4.80 M, if the reaction has a half life of 125 sec, what is the concentration at 360. second? 0.454 M 1.24 M 0.897 M 2.25 M Cannot determine, not enough information provided. Submit
* 97% 7:57 PM Wed Feb 12 х Unanswered •1 attempt left • Due on Feb 13 The initial concentration of reactant of a 2nd order reaction is 4.80 M, if the reaction has a half life of 125 sec, what is the concentration at 360. second? 0.454 M 1.24 M 0.897 M 2.25 M Cannot determine, not enough information provided. Submit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 22QAP: The hypothetical reaction X(g)+12Y(g)productsis first-order in X and second-order in Y. The rate of...
Related questions
Question
Transcribed Image Text:* 97%
7:57 PM Wed Feb 12
х
Unanswered •1 attempt left • Due on Feb 13
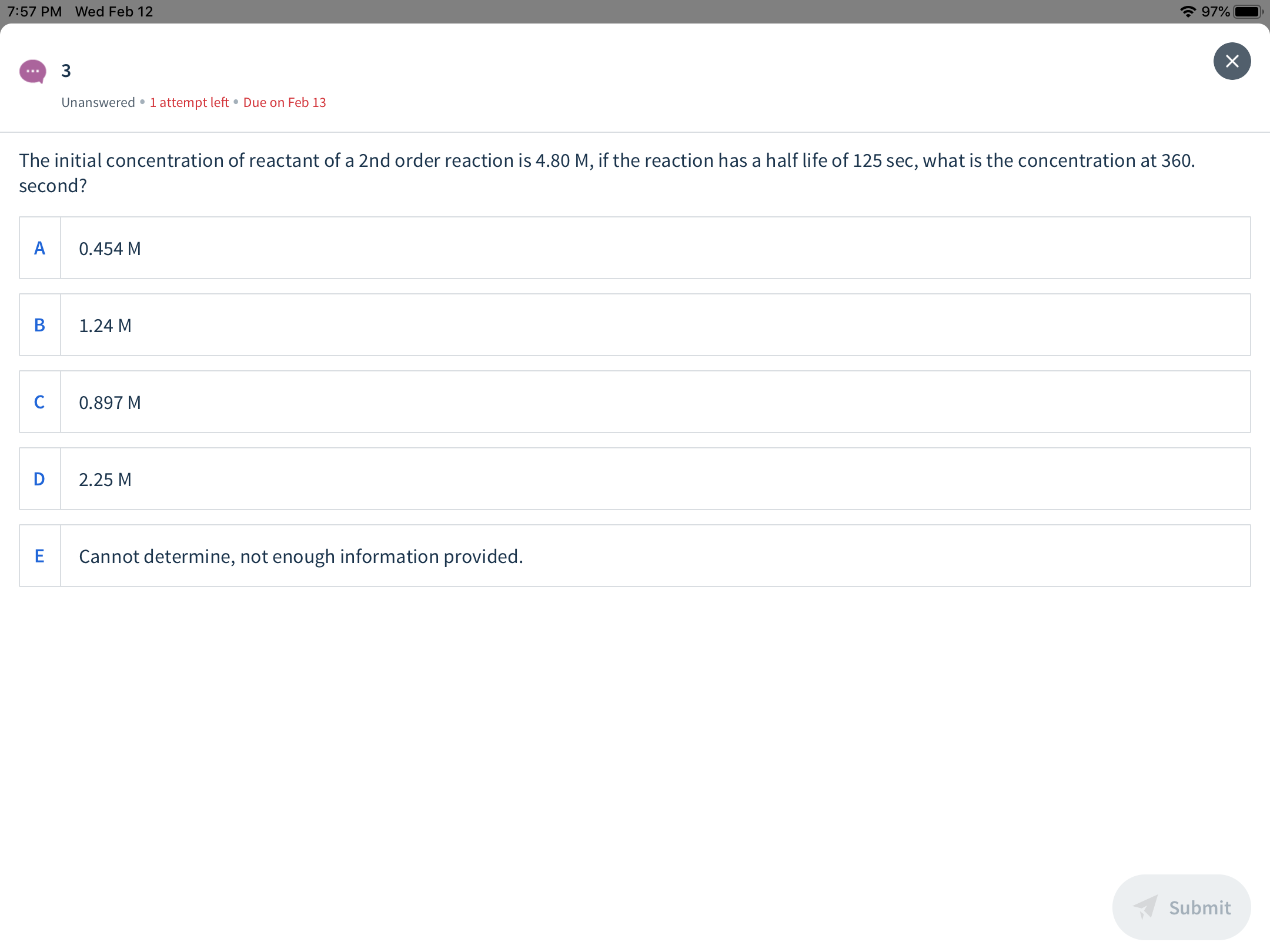
The initial concentration of reactant of a 2nd order reaction is 4.80 M, if the reaction has a half life of 125 sec, what is the concentration at 360.
second?
0.454 M
1.24 M
0.897 M
2.25 M
Cannot determine, not enough information provided.
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning