* 97% 7:58 PM Wed Feb 12 х Unanswered •1 attempt left • Due on Feb 13 When the initial concentration of the reactant increases, the half life of a 1st order reaction will and the half life of a 2nd order reaction will increase, decrease B not change, decrease decrease, increase not change, increase increase, not change Submit ш
* 97% 7:58 PM Wed Feb 12 х Unanswered •1 attempt left • Due on Feb 13 When the initial concentration of the reactant increases, the half life of a 1st order reaction will and the half life of a 2nd order reaction will increase, decrease B not change, decrease decrease, increase not change, increase increase, not change Submit ш
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.20E: In each of the following, which reaction mechanism assumption is apparently being violated? Explain...
Related questions
Question
Transcribed Image Text:* 97%
7:58 PM Wed Feb 12
х
Unanswered •1 attempt left • Due on Feb 13
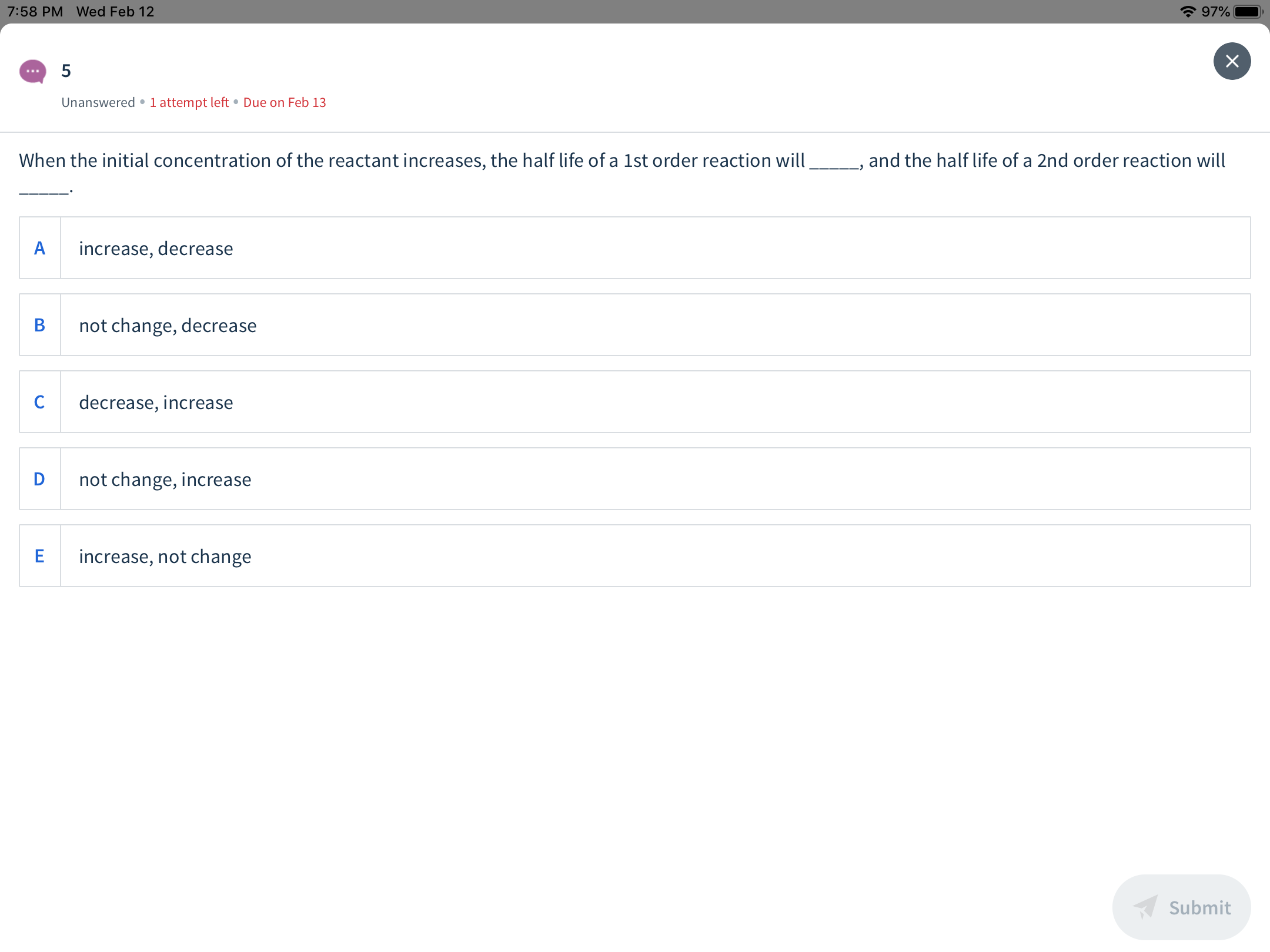
When the initial concentration of the reactant increases, the half life of a 1st order reaction will
and the half life of a 2nd order reaction will
increase, decrease
B
not change, decrease
decrease, increase
not change, increase
increase, not change
Submit
ш
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning