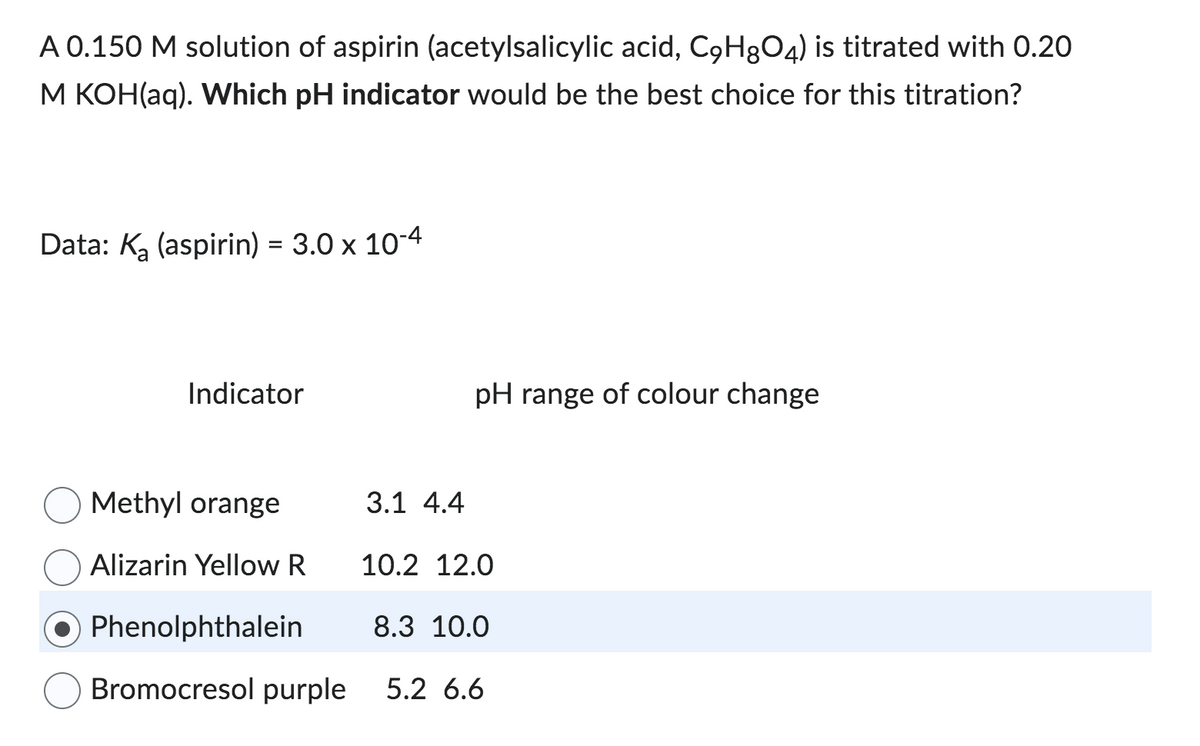
A 0.150 M solution of aspirin (acetylsalicylic acid, C9H8O4) is titrated with 0.20 M KOH(aq). Which pH indicator would be the best choice for this titration? Data: K₂ (aspirin) = 3.0 x 10-4 Indicator Methyl orange Alizarin Yellow R 3.1 4.4 pH range of colour change 10.2 12.0 Phenolphthalein Bromocresol purple 5.2 6.6 8.3 10.0
Q: Nat - Hi + t →Nat X Pact A: Identify the lewis acids, bases; determine if they are bronted ac pact…
A: Since you have asked multiple questions, we will answer only first question for you. In order to get…
Q: 4. H3C H3C 5. CH3 CH3
A: IUPAC nomenclature: In IUPAC nomenclature a molecule's longest chain of carbons is taken as parent…
Q: 2 n Ool + CI + CI AICI, -CH3 + Br₂ hv AICI
A: Friedel-Crafts alkylation and acylation reactions are organic reactions used to introduce an alkyl…
Q: Nitrogen gas reacts with oxygen gas to form nitrogen monoxide gas. Write a balanced chemical…
A: Chemical formula nitrogen gas. N2 (g) Oxygen gas…
Q: Problem 2. Propose the major products for the following reactions. 1. 2. 3. Br + (CH₂CH₂CH₂CH₂)₂Culi…
A: In these three questions, we will find the major organic product. First reaction is nucleophilic…
Q: 5-Chloro-1,3-cyclopentadiene (below) undergoes SN1 solvolysis in the presence of silver ion ex…
A: Solvolysis is a type of chemical reaction in which a substrate molecule is dissolved in a solvent…
Q: A. Complete the table below. Use your knowledge on scientific notation and unit prefixes to complete…
A: Scientific notation is a way of expressing numbers in a more compact form. In scientific notation,…
Q: What are some general and specific implications of using titrations to identify unknown acids?
A: Titration is a laboratory technique used to determine the concentration of a solution of known…
Q: The equilibrium boiling point of ethanol, CH3CH₂OH, is T = 78°C at P = 1.00 atm. What are the signs…
A: Explanation For non spontaneous process ΔG > 0 and ΔSuniv < 0 For spontaneous process ΔG <…
Q: Draw the line form into condensed formula. H₂C CH3 CH₂ CH3 CH3 CH3 CH3
A:
Q: EQUATION CLASSIFICATIONS: combustion, synthesis, decomposition, single-displacement,…
A:
Q: Predict the splitting pattern expected for the circled proton in the structure below. Consider…
A:
Q: ОН POCIĄ pyridine
A: In presence of POCl3 and pyridine there is removel of Water and double bond formed.
Q: Step 2: In a reply below, answer the following prompt. Be sure to respond to the thoughts of your…
A: Solutions- The correct option is 1 or A. A decrease in weight from chicken piece 1 to 4.
Q: A sample of gourmet table salt has a mass of 228 g and a volume of 102 cm³ (as determined by the…
A:
Q: unbalanced equation is B₂S3 + H₂O → H,BO3 + H₂S [For each compound, construct a vector that lists…
A: •Given:-
Q: A yellow balloon is filled with 0.5 mol of Ne and a red balloon is filled with 1.0 mol of Ar. Both…
A: Ideal gas equation given as PV = nRT P= pressure, V= volume, T= temprature n= moles, R = gas…
Q: The IR spectrum below reveals what functional group, if any? give explanation for your choice.
A: IR (Infrared) spectroscopy deal with measurement of the interaction of infrared radiation with…
Q: At a certain temperature the rate of this reaction is second order in N₂O5 with a rate constant of…
A:
Q: Calculate the pH of the resulting solution if 33.0 mL of 0.330 M HCl(aq) is added to 43.0 mL of…
A: •As you have posted multiple questions so we are answering first question for you according to…
Q: Decomposition of NH3 in presence of molybdenum is a zero-order reaction with a rate constant of…
A: The reaction in which the rate of the reaction does not depend on the concentration of reactant is…
Q: 859g=Mg
A: given 859 g we have to convert it into mg
Q: What is the pH of the following solutions? 10 mM H2SO4 80 mM TRIS base
A: pH of a solution indicates the concentration of -OH or H+ ions. It can be calculated by using the…
Q: Assume that drug X has a chemical makeup that results in its being water-soluble and that of drug Y…
A: •Given:- Drug X => Water soluble Drug Y => fat soluble
Q: Calculate the root mean square (rms) average speed of the atoms in a sample of argon gas at 0.12 atm…
A: Using formula for root mean square speed formula.
Q: 8. Identify the species that results from the movement of the following elelctron pairs. H N:
A: Answer:- This question is answered by using the simple concept of writing the resonating structure…
Q: 2 3 5 6 Predicate the products of each reaction: Aldehydes + Ketones CH;CH + HC=N o O CH₂-C-OCH, =O…
A: Here, we have to draw the products for the given organic reactions.
Q: What is the rate for the second order reaction A → Products when [A] = 0.260 M? (k = 0.761 M¯'s¯¹)
A:
Q: Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right).…
A:
Q: What is the IUPAC name for the following compound? CH₂ CH₂CH₂CH₂ CH₂-C-CH₂-CH-CH₂ CH₂CH₂
A:
Q: If air in a sealed flask at 25 degrees C has a pressure of 147.2 KPa, what is the value of the…
A: Given Pressure= 147.2 kpa Temperature= 25°C
Q: Air in a sealed flask at 23.5 degrees C has a pressure of 102.8 KPa. What is the pressure (in KPa)…
A:
Q: 10. Compound A (s) is decomposed into B (g) and C (g). Equilibrium constant of this reaction is…
A:
Q: A self-contained underwater breathing apparatus uses canisters containing potassium superoxide. The…
A: Note: As per guidelines solution of first question has been made. For the expert solution of second…
Q: 1d) What are the expected NMR and IR for the following compound?
A: For all the types of different bonds there will be different signal in IR spectrum. Similarly for…
Q: At 2000 °C the equilibrium constant for the reaction 2NO(g) = N₂(g) + O2(g) is Ke 2.4 x 10³. ▼ Part…
A:
Q: Calculate the percent yield and theoretical yield for the following reaction: oxidation of…
A: Given data 0.2093 grams of Cyclododecanol 0.5 mL of acetone 0.20 mL of Glacial acetic acid 2.0 mL…
Q: What is the concentration of A after 23.1 minutes for the reaction A → Products when the initial…
A: By identifying the order of reaction and using integrated rate equation we can identify the…
Q: Supposing the stock room wants to prepare 50.0 mL a 0.080 M solution of oxalic acid (H2C2O4).…
A: Given Volume = 50.0 ml Molarity = 0.080 M
Q: If the starting concentration of CO2 is 0 M, and the reaction rate for the first 10 seconds is 0.060…
A: The rate of appearance of the product is equal to the change in concentration of the product divided…
Q: Fill in the name and empirical formula of each ionic compound that could be formed from the ions in…
A: •Given:-
Q: it says to only use 2 significant figures. please try again.
A: Ideal gas equation is PV = nRT Where, P is pressure of the gas V is volume of the gas n is no. of…
Q: How much of 6M HCl would you need to add if you have 800 ml of 100 mM TRIS (ph of 10.2) and you want…
A: Given, Molarity of HCl from stock,M1 = 6M Volume of HCl from stock, V1 = ? Molarity of TRIS…
Q: What is the IUPAC name of the following structure? O 1-methyl-6-methylcyclohexene O…
A: For the first question, we have to write the IUPAC name of the given organic compound. For the…
Q: An organic compound is insoluble in water and sulfuric acid. It gave a negative sodium fusion test,…
A: Organic compounds insoluble in water and sulphuric acid are non-polar with no labile H to form H…
Q: cation Ca²+ 3+ Mn 2+ Ba Cu anion NO₂ 104 BrO PO some ionic compounds empirical formula Ca(NO₂)₂ Mn…
A: We can write the names of ionic compounds from their cations and anions. We write cation first and…
Q: f) What are the expected NMR and IR for the following compound?
A: 1H NMR provides the information of hydrogen environment of the unknown compound. 13C-NMR provides…
Q: For the following reaction, 28.3 grams of sulfur dioxide are allowed to react with 6.16 grams of…
A: Given : mass of sulfur dioxide = 28.3 g Mass of oxygen = 6.16 g
Q: a solutipn prepared by dissolving 6.95 x 10^-3 g of protein in 0.0300L of water has an osmotic…
A:
Q: What molarity of HCI stock solution is needed so that 2.5mL of this stock solution diluted to 0.025L…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- Chitinase is a protein that breaks down chitin, a primary component of the cell wall in fungi, scales in fish and exoskeletons of arthropods. The activity of chitinase extracted from a plant was shown to be optimum at pH 5. You were tasked to prepare 300 mL of 150 mM buffer solution for further analysis of the extracted chitinase. REAGENTS Ka 2.5M Acetic acid Solid NaOAc•3H2O [136.08g/mol] 1.76 x 10-5 2.5M NH3 Solid NH4Cl [53.49g/mol] 5.6 x 10-10 2.5M Lactic acid Solid sodium lactate [112.06g/mol] 4.0 x 10-5 5 M HCl 5M NaOH What are the moles of the (1) acid and (2) base components, given the following reagents? What is the mass/volume of the components needed to prepare the buffer solution?The SO2 present in air is mainly responsible for the phenomenon of acid rain. The concentration of SO2 can be determined by titrating against a standard permanganate solution as follows: 5SO2 + 2MnO4- + 2H2O ---> 5SO42- + 2Ms2 + 4H+ Calculate the number of grams of SO2 in a sample of air if 4.90mL of 0.00700M KMnO4 solution are required for the titration. Be sure your answer has the correct number of significant digits.134 grams of potassium sorbate KCH3 (CH)4CO2 is fully dissolved in 100.00 mL of water, which is carefully transferred to a conical flask. Then 100.00 mL of 0.240 M HNO3 is added dropwise to this solution from a burette. Given: Ka (sorbic acid) = 1.7 × 1O^-5 Showing all your calculations and reasoning, determine the pH of the solution that results after the addition of all the acid mentioned above. Suppose that the titration continues. Determine the pH of the solution in the flask at theequivalence point
- Calculate the molar solubility of calcium oxalate in a solution that has been buffered so that its pH is constant and equal to 4.00. Data: Kps of CaC2O4 = 1.7x10 -9 ; Ka1 of H2C2O4 = 5.60x10-2 and Ka2 = 5.42x10-5 . Use systematic treatment.The protein content of wheat flour can be determined reasonably accurately by multiplying the percentage of nitrogen present by 5.7. A 2.06-g sample of flour was taken through a Kjeldahl procedure and the ammonia produced was distilled into a boric acid solution. If this solution required 34.70 mL of 0.174 N HCl for titration to the methyl red end point, what is the a) % Nitrogen and b) % protein in the flour? (Use 1:1 stoich ratio between N and HCl)A 20.00 mL aliquot of lactic acid solution (HCH3H5O3) was titrated with 0.0980 M KOH(aq) using both an indicator and a pH meter. Ka (HCH3H5O3), is 1.38 x10-4. A total of 28.64 mL of 0.0980 M KOH(aq) was required to reach the equivalence point 1. Calculate the molarity of the lactic acid solution. 2. Calculate the pH of the lactic acid solution 3. Calculate the pH and [CH3H5O3-] at the half-equivalence point. 4. Calculate the pH at the equivalence point of the titration. 5. Suggest an appropriate indicator for titration. 6. Calculate the pH of the solution after 10.00 mL of 0.0980 M NaOH(aq) was added
- A metal M forms a water-soluble hydroxide with a chemical formula of MOH. To determine whatM is, a student prepared 250.0 cm3 of MOH standard solution by dissolving 1.17 g of MOH in distilled water. Then the student titrated 25.0 cm3of the solution with 0.055 M H2SO4(aq) usingphenolphthalein as indicator. The titration was repeated several times and the mean titre was 18.85cm3.(a) Describe how the 250.0 cm3 of MOH standard solution was prepared. (b) (i) Calculate the molar mass of MOH. (ii) Determine what M is. (Relative atomic masses: H = 1.0, O = 16.0) THANKYOU!!!Use the following experimental titration data to calculate the concentration of the acid being analysed. Observations: The initial solution of acetic acid (HC2H3O2) is clear and colourless. A few drops of phenolphthalein indicator are added to each sample. A dilute solution of sodium hydroxide (concentration 2.50 x 10-4 mol/L) is used as the titrant. As the mixture reaches the endpoint, flashes of pink colour are seen and the titrant is added drop by drop. The endpoint is reached when one drop of titrant turns the mixture a pale pink colour that does not fade.What is the difference between alkalinity and buffering capacity?
- A 25.0-mL aliquot of vinegar was diluted to 250 mL in a volumetric flask. Titration of 50.0-mL aliquots of the diluted solution required an average of 25.23 mL of 0.09041 M NaOH. Express the acidity of the vinegar in terms of the percentage (w/v) of acetic acid.Determine the pH at the equivalence (stoichiometric) point in the titration of 38.83 mL of 0.262 M (CH3)2NH(aq) with 0.111 M HCl(aq). The Kb of (CH3)2NH is 5.4 x 10-4.You have a solution that contains acetic acid, (CH3COOH or HAc). The concentration of acetic acid in the aqueous solution is 0.35 mol / dm3. Ka = 1.7378 x 10 ^ -5 M a. Calculate the pH of the solution. 15 cm 3 of the acetic acid solution was titrated with sodium hydroxide solution, NaOH (aq), at a concentration of 0.5 mol / dm3 b. Describe how you would proceed to perform a titration of an acid of unknown concentration. What information can be obtained from a titration, describe the different phases. c. How much NaOH will be added when the equivalence point occurs?

