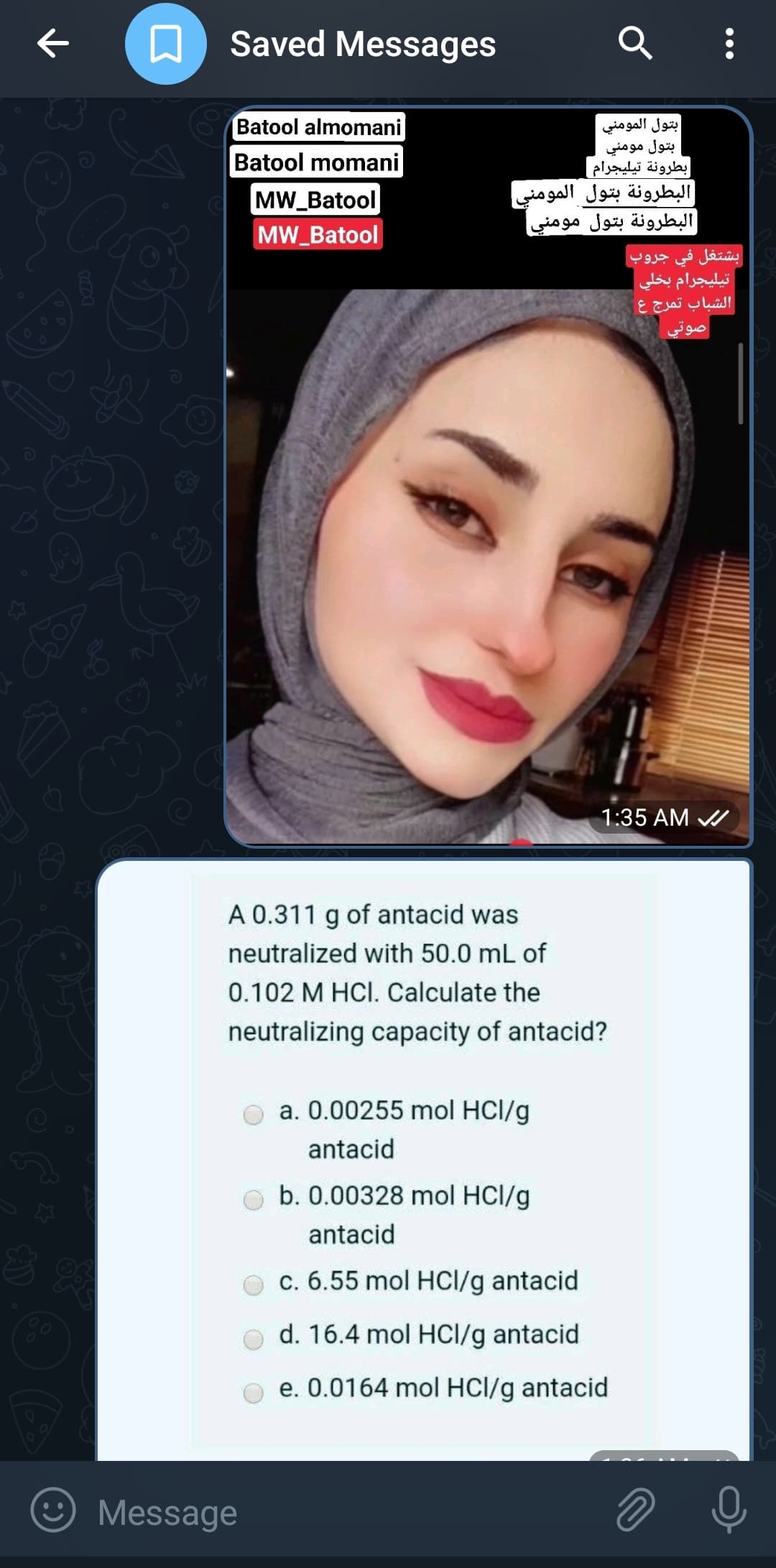
A 0.311 g of antacid was neutralized with 50.0 mL of 0.102 M HCI. Calculate the neutralizing capacity of antacid? a. 0.00255 mol HCl/g antacid b. 0.00328 mol HCl/g antacid c. 6.55 mol HCl/g antacid d. 16.4 mol HCl/g antacid e. 0.0164 mol HCl/g antacid
A 0.311 g of antacid was neutralized with 50.0 mL of 0.102 M HCI. Calculate the neutralizing capacity of antacid? a. 0.00255 mol HCl/g antacid b. 0.00328 mol HCl/g antacid c. 6.55 mol HCl/g antacid d. 16.4 mol HCl/g antacid e. 0.0164 mol HCl/g antacid
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section23.5: Basicity
Problem GQ
Related questions
Question
Transcribed Image Text:ه
ی
03
A
سد
Saved Messages
Batool almomani
Batool momani
| MW_Batool
MW_Batool
Message
بتول المومني
بتول مومني
بطرونة تيليجرام |
البطرونة بتول المومني
البطرونة بتول مومني
A 0.311 of antacid was
neutralized with 50.0 mL of
0.102 M HCI. Calculate the
neutralizing capacity of antacid?
a. 0.00255 mol HCl/g
antacid
Q :
11:35 AM
b. 0.00328 mol HCl/g
antacid
c. 6.55 mol HCl/g antacid
d. 16.4 mol HCl/g antacid
e. 0.0164 mol HCl/g antacid
بشتغل في جروب
تيليجرام بخلي
الشباب تمرج ع
صوتي
ه
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax