A 0.357 mol sample of N, (g), initially at 298 K and 1.00 atm, is held at constant pressure while enough heat is applied to raise the temperature of the gas by 12.3 K. Calculate the amount of heat q required to bring about this temperature change, and find the corresponding total change in the internal energy AU of the gas. Assume that the constant-pressure molar specific heat for N, (g), which consists of linear molecules, is equal to 7R/2, where R = 8.3145 J/(mol-K) is the ideal gas constant.
A 0.357 mol sample of N, (g), initially at 298 K and 1.00 atm, is held at constant pressure while enough heat is applied to raise the temperature of the gas by 12.3 K. Calculate the amount of heat q required to bring about this temperature change, and find the corresponding total change in the internal energy AU of the gas. Assume that the constant-pressure molar specific heat for N, (g), which consists of linear molecules, is equal to 7R/2, where R = 8.3145 J/(mol-K) is the ideal gas constant.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 66AP
Related questions
Question
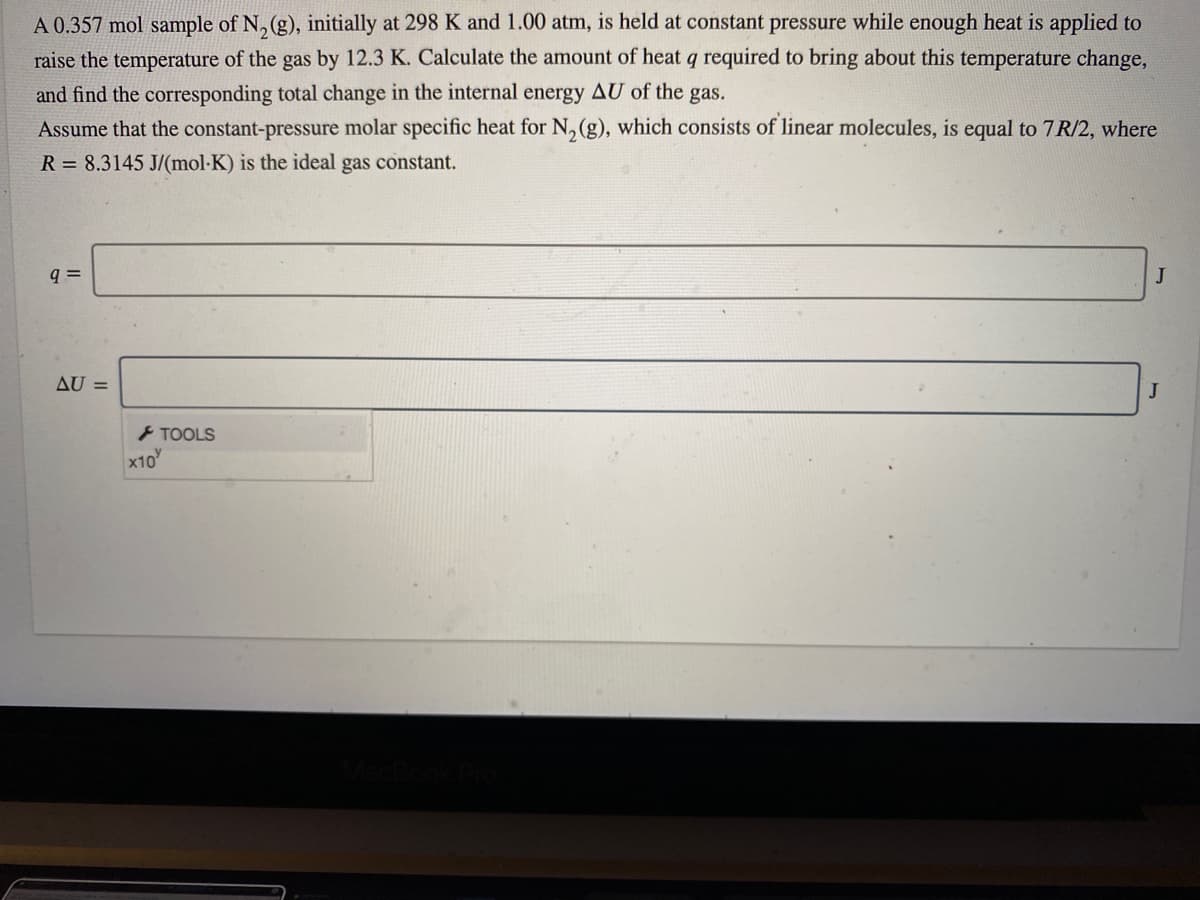
Transcribed Image Text:A 0.357 mol sample of N, (g), initially at 298 K and 1.00 atm, is held at constant pressure while enough heat is applied to
raise the temperature of the gas by 12.3 K. Calculate the amount of heat q required to bring about this temperature change,
and find the corresponding total change in the internal energy AU of the gas.
Assume that the constant-pressure molar specific heat for N, (g), which consists of linear molecules, is equal to 7R/2, where
R = 8.3145 J/(mol-K) is the ideal gas constant.
q =
J
AU =
J
> TOOLS
x10
Expert Solution
Step 1
Given ,
Number of moles
Temperature
Pressure
R
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax