A 100% 7:06 PM Wed Jan 29 Unanswered •1 attempt left • Due on Jan 30 0.100 g sample is dissolved in 10.0 g of a solvent (Kf = 20.0 kg °C/mol), If the freezing point of the solution is found to be 1.22°C lower than the freezing point of pure solvent, the molecular weight of the sample is -g/mol. 328 0.610 164 244 1.64 Submit
A 100% 7:06 PM Wed Jan 29 Unanswered •1 attempt left • Due on Jan 30 0.100 g sample is dissolved in 10.0 g of a solvent (Kf = 20.0 kg °C/mol), If the freezing point of the solution is found to be 1.22°C lower than the freezing point of pure solvent, the molecular weight of the sample is -g/mol. 328 0.610 164 244 1.64 Submit
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 45P
Related questions
Question
Transcribed Image Text:A 100%
7:06 PM Wed Jan 29
Unanswered •1 attempt left • Due on Jan 30
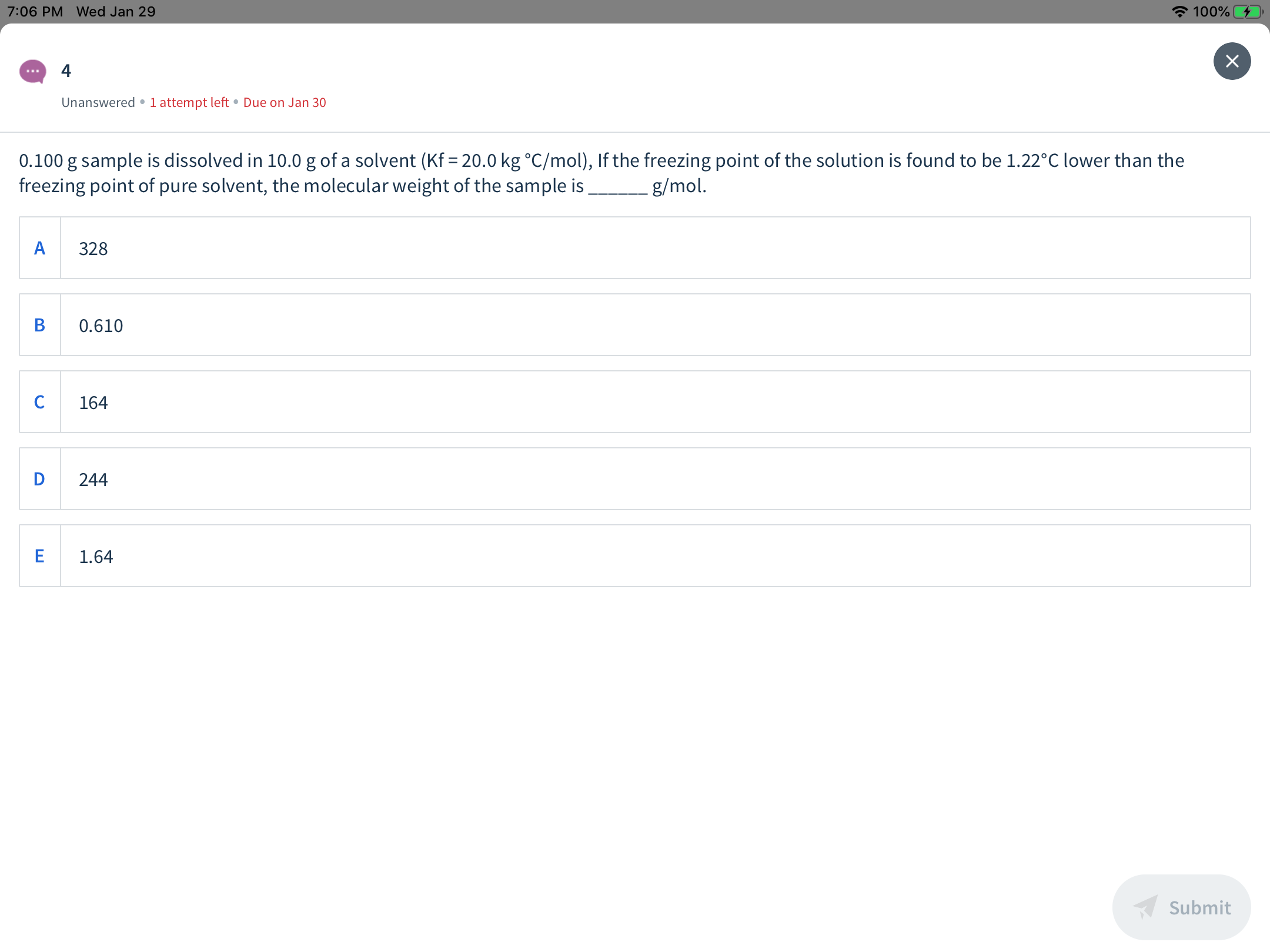
0.100 g sample is dissolved in 10.0 g of a solvent (Kf = 20.0 kg °C/mol), If the freezing point of the solution is found to be 1.22°C lower than the
freezing point of pure solvent, the molecular weight of the sample is
-g/mol.
328
0.610
164
244
1.64
Submit
Expert Solution
Step 1
Mass of solute = 0.100 g
Mass of solvent = 10.0 g
Depression in freezing point (ΔTf) = 1.22 °C
freezing point depression constant (Kf) = 20.0 kg. °C/mol
Step 2
Colligative properties are the properties of the solution that depend on the number of moles of solute. These properties are different from the solute particles. These properties include boiling point elevation, freezing point depression, osmotic pressure, and vapor pressure lowering.
Step 3
The change in freezing point is calculated by the formula as shown below:

Step by step
Solved in 6 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning