A 100% 7:06 PM Wed Jan 29 Unanswered •1 attempt left • Due on Jan 30 When a non-volatile solute is dissolved in a solvent, the boiling point of the solution and the vapor pressure of the solution Decreases; decreases B Decreases; increases Increases; increases Increases; remain the same Increases; decreases Submit ш
A 100% 7:06 PM Wed Jan 29 Unanswered •1 attempt left • Due on Jan 30 When a non-volatile solute is dissolved in a solvent, the boiling point of the solution and the vapor pressure of the solution Decreases; decreases B Decreases; increases Increases; increases Increases; remain the same Increases; decreases Submit ш
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 78QAP: A gaseous solute dissolves in water. The solution process has H=15 kJ. Its solubility at 22C and...
Related questions
Question
Transcribed Image Text:A 100%
7:06 PM Wed Jan 29
Unanswered •1 attempt left • Due on Jan 30
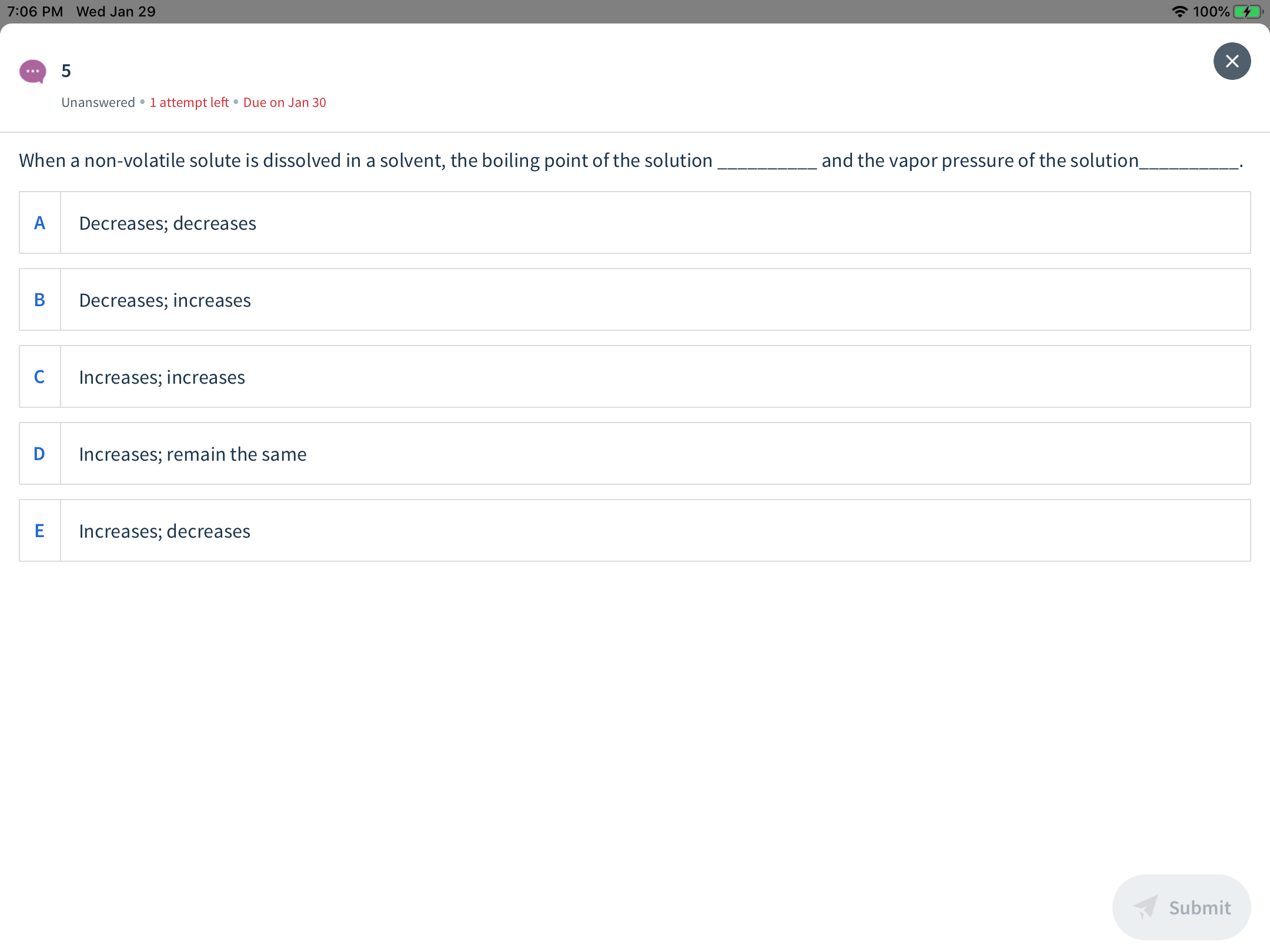
When a non-volatile solute is dissolved in a solvent, the boiling point of the solution
and the vapor pressure of the solution
Decreases; decreases
B
Decreases; increases
Increases; increases
Increases; remain the same
Increases; decreases
Submit
ш
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT