A 47.0-g sample of copper at 99.9 °C is dropped into a beaker containing 157 g of water at 19.0 °C. What is the final temperature when thermal equilibrium is reached? (The specific heat capacities of liquid water and copper are 4.184 J/g K and 0.385 J/g K, respectively.)
A 47.0-g sample of copper at 99.9 °C is dropped into a beaker containing 157 g of water at 19.0 °C. What is the final temperature when thermal equilibrium is reached? (The specific heat capacities of liquid water and copper are 4.184 J/g K and 0.385 J/g K, respectively.)
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem SI6RE
Related questions
Question
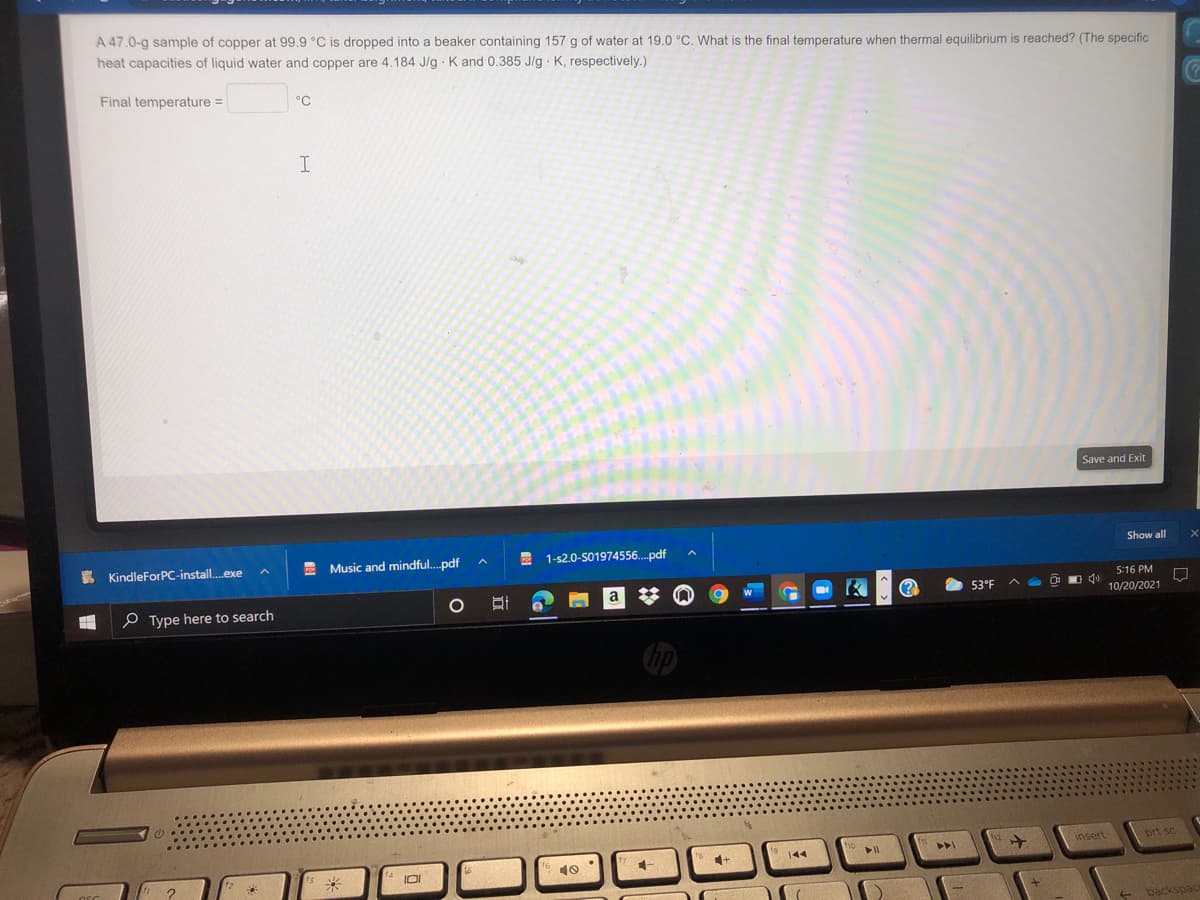
Transcribed Image Text:A 47.0-g sample of copper at 99.9 °C is dropped into a beaker containing 157 g of water at 19.0 °C. What is the final temperature when thermal equilibrium is reached? (The specific
heat capacities of liquid water and copper are 4.184 J/g K and 0.385 J/g K, respectively.)
Final temperature =
°C
Save and Exit
E KindleForPC-install.exe
2 Music and mindful.pdf
2 1-s2.0-S01974556.pdf
Show all
5:16 PM
53°F
e Type here to search
10/20/2021
insert
prt sc
144
10
backspac
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning