A buffer is prepared by mixing 525 mL of 0.50 M HCHO2 and 475 mL of 0.50 M NaCHO2. Calculate the pH. What would be the pH of 85 mL of the buffer to which 8.6 mL of HCl has been added? The Ka of HCHO2 = 1.8 x 10-4 Molarity for HCI is 0.8M
A buffer is prepared by mixing 525 mL of 0.50 M HCHO2 and 475 mL of 0.50 M NaCHO2. Calculate the pH. What would be the pH of 85 mL of the buffer to which 8.6 mL of HCl has been added? The Ka of HCHO2 = 1.8 x 10-4 Molarity for HCI is 0.8M
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter16: Acid-base Equilibria
Section: Chapter Questions
Problem 16.78QP: A buffer is prepared by mixing 525 mL of 0.50 M formic acid, HCHO2, and 475 mL of 0.50 M sodium...
Related questions
Question
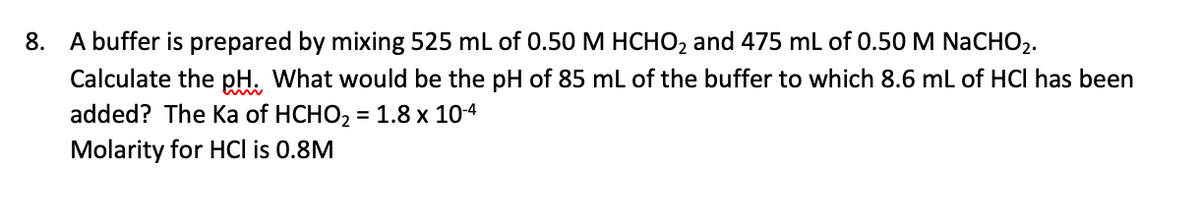
Transcribed Image Text:8. A buffer is prepared by mixing 525 ml of 0.50 M HCHO2 and 475 mL of 0.50 M NaCHO2.
Calculate the pH. What would be the pH of 85 mL of the buffer to which 8.6 mL of HCI has been
added? The Ka of HCHO, = 1.8 x 104
Molarity for HCl is 0.8M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning