a) Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. kJ/mol b) Given your value in (a), calculate the percent of the chair, indicated as B, presented in an equilibrium mixture of the conformers at 25°C. %-
a) Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign. kJ/mol b) Given your value in (a), calculate the percent of the chair, indicated as B, presented in an equilibrium mixture of the conformers at 25°C. %-
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.SE: Something Extra
Problem 24VC: A trisubstituted cyclohexane with three substituents-red, green, and blue-undergoes a ring-flip to...
Related questions
Question
i need help
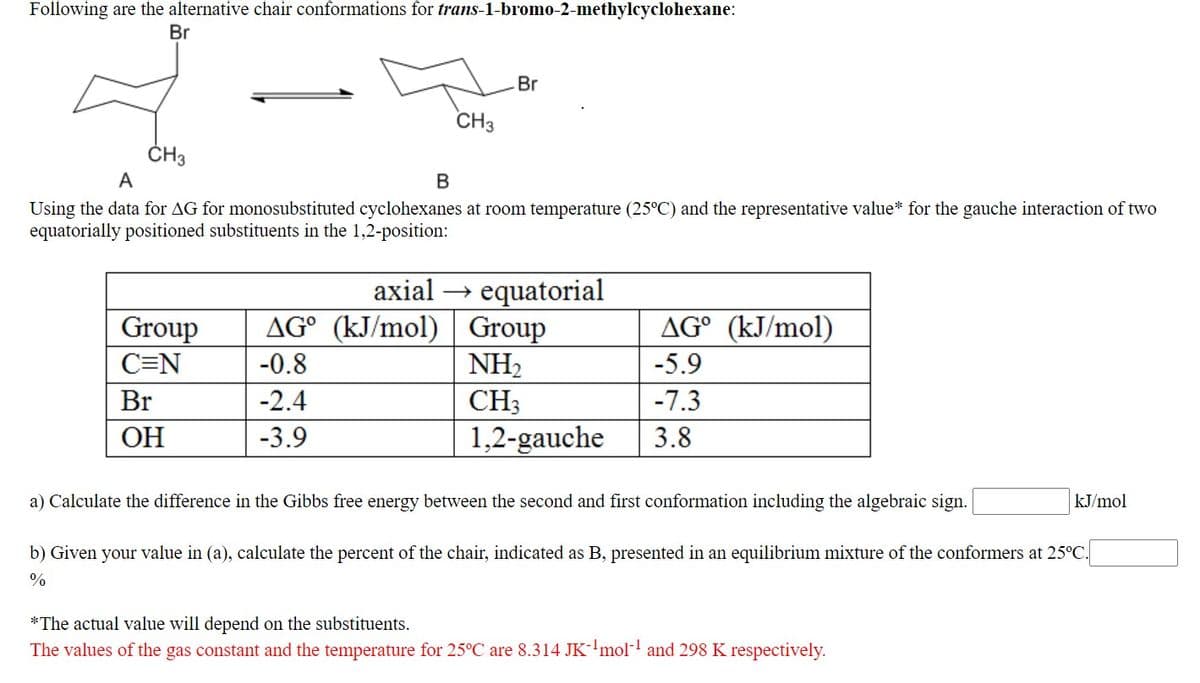
Transcribed Image Text:Following are the alternative chair conformations for trans-1-bromo-2-methylcyclohexane:
Br
Br
CH3
ČH3
А
В
Using the data for AG for monosubstituted cyclohexanes at room temperature (25°C) and the representative value* for the gauche interaction of two
equatorially positioned substituents in the 1,2-position:
axial → equatorial
AG° (kJ/mol) | Group
NH2
Group
AG° (kJ/mol)
C=N
-0.8
-5.9
Br
-2.4
CH3
-7.3
ОН
-3.9
1,2-gauche
3.8
a) Calculate the difference in the Gibbs free energy between the second and first conformation including the algebraic sign.
kJ/mol
b) Given your value in (a), calculate the percent of the chair, indicated as B, presented in an equilibrium mixture of the conformers at 25°C.
%
*The actual value will depend on the substituents.
The values of the gas constant and the temperature for 25°C are 8.314 JK-'mol- and 298 K respectively.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning