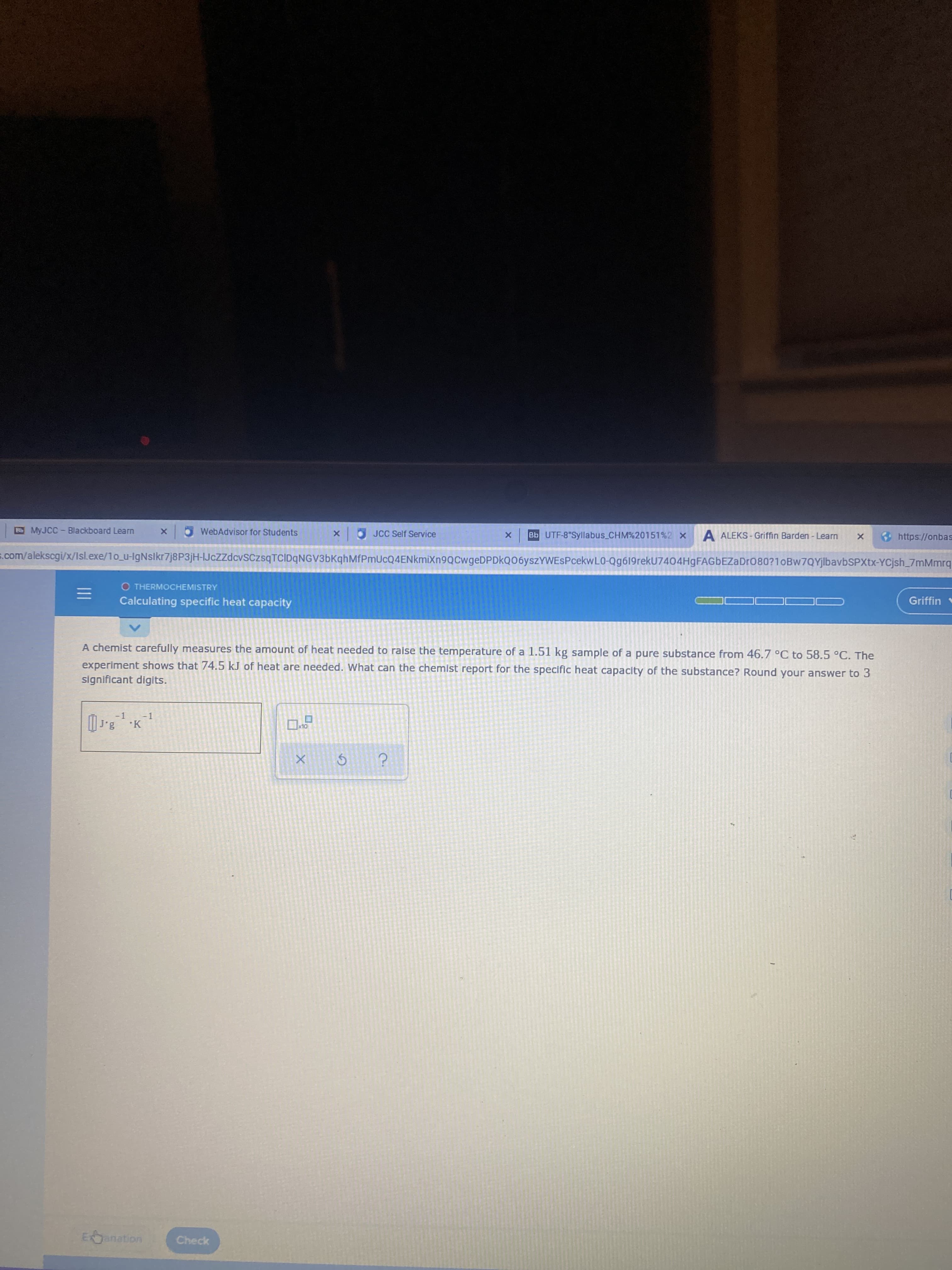
A chemist carefully measures the amount of heat needed to raise the temperature of a 1.51 kg sample of a pure substance from 46.7 °C to 58.5 °C. The experiment shows that 74.5 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3 significant digits.
A chemist carefully measures the amount of heat needed to raise the temperature of a 1.51 kg sample of a pure substance from 46.7 °C to 58.5 °C. The experiment shows that 74.5 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3 significant digits.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:Bb MYJCC- Blackboard Learn
WebAdvisor for Students
O JCC Self Service
Bb UTF-8 Syllabus_CHM%20151%2 X
A ALEKS - Griffin Barden - Learn
https://onbas
s.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg619rekU7404HgFAGbEZaDr080?1oBw7QYjlbavbSPXtx-YCjsh_7mMmrq
O THERMOCHEMISTRY
Griffin
Calculating specific heat capacity
A chemist carefully measures the amount of heat needed to raise the temperature of a 1.51 kg sample of a pure substance from 46.7 °C to 58.5 °C. The
experiment shows that 74.5 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3
significant digits.
-1 -1
Exanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY