A chemist carefully measures the amount of heat needed to raise the temperature of a 126.0 g sample of a pure substance from -2.8 °C to 13.3 °C. The experiment shows that 911. J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3 significant digits. -1 -1
A chemist carefully measures the amount of heat needed to raise the temperature of a 126.0 g sample of a pure substance from -2.8 °C to 13.3 °C. The experiment shows that 911. J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3 significant digits. -1 -1
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
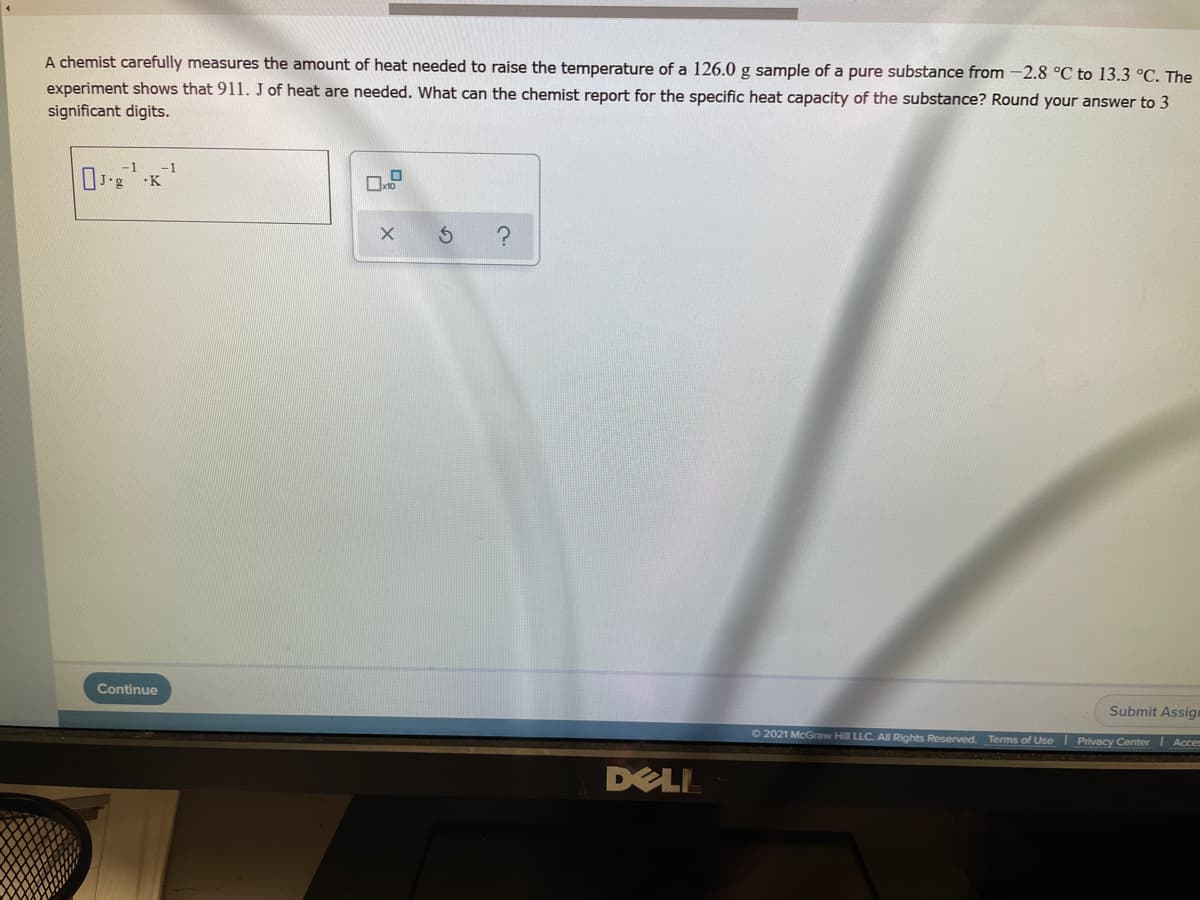
Transcribed Image Text:A chemist carefully measures the amount of heat needed to raise the temperature of a 126.0 g sample of a pure substance from -2.8 °C to 13.3 °C. The
experiment shows that 911. J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3
significant digits.
-1
-1
Continue
Submit Assig
62021 McGraw Hill LLC. All Rights Reserved. Terms of Use
Privacy Center Acces
DEL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning