A chemist prepares a solution of copper(II) fluoride (CuF2) by measuring out 1.4 mg of copper(II) fluoride into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's copper(II) fluoride solution. Be sure your answer has the correct number of significant digits. mol/L x10 ? X Submit Assignment Terms of Use Privacy 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro DD O00 F10 F 9 F8 F 7 F6 F 5 F 4 F3 F2 F1 %0 5 3 * co
A chemist prepares a solution of copper(II) fluoride (CuF2) by measuring out 1.4 mg of copper(II) fluoride into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's copper(II) fluoride solution. Be sure your answer has the correct number of significant digits. mol/L x10 ? X Submit Assignment Terms of Use Privacy 2019 McGraw-Hill Education. All Rights Reserved. MacBook Pro DD O00 F10 F 9 F8 F 7 F6 F 5 F 4 F3 F2 F1 %0 5 3 * co
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
ChapterA: Scientific Notation And Experimental Error
Section: Chapter Questions
Problem 10P
Related questions
Question
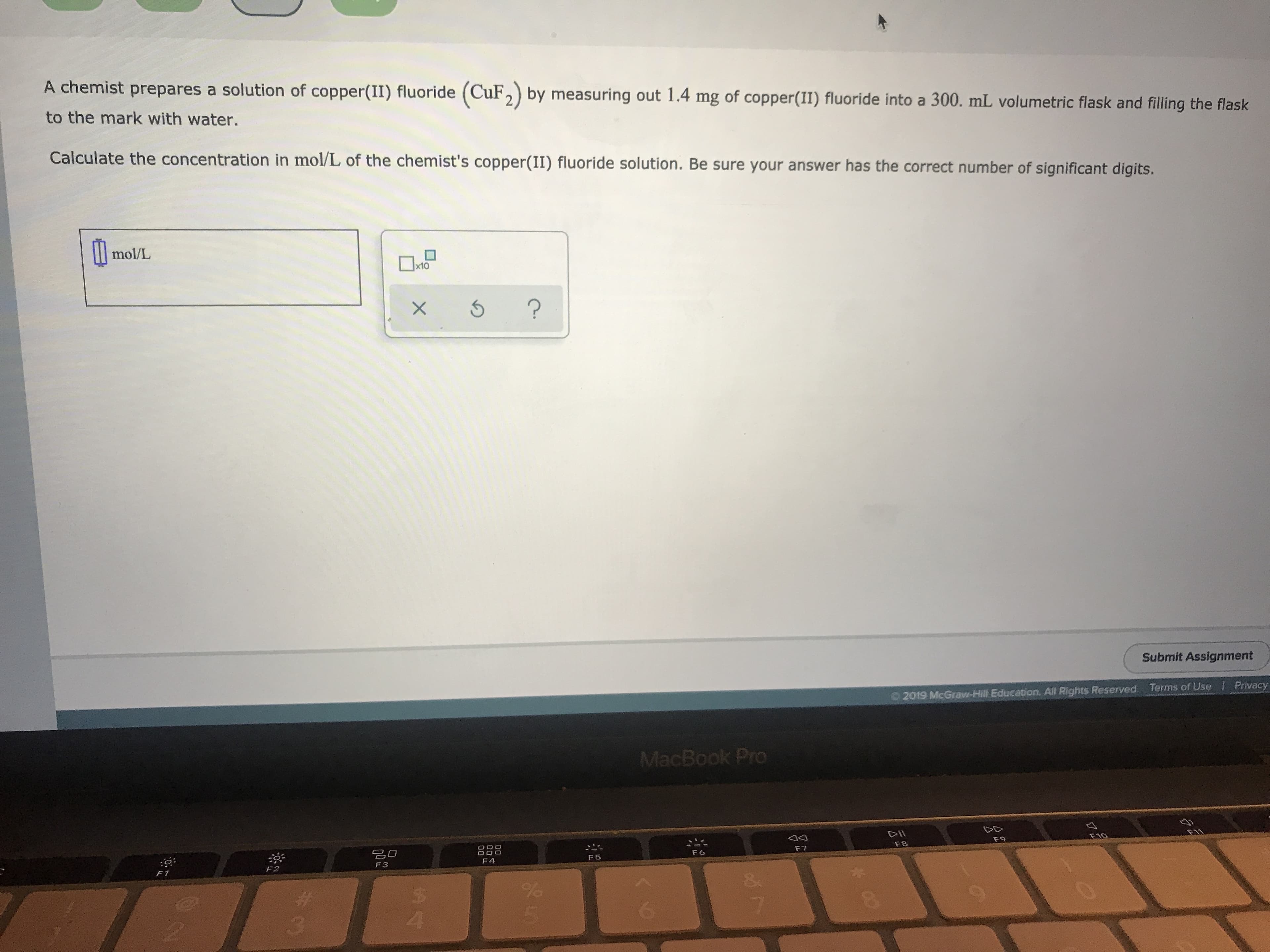
Transcribed Image Text:A chemist prepares a solution of copper(II) fluoride (CuF2) by measuring out 1.4 mg of copper(II) fluoride into a 300. mL volumetric flask and filling the flask
to the mark with water.
Calculate the concentration in mol/L of the chemist's copper(II) fluoride solution. Be sure your answer has the correct number of significant digits.
mol/L
x10
?
X
Submit Assignment
Terms of Use
Privacy
2019 McGraw-Hill Education. All Rights Reserved.
MacBook Pro
DD
O00
F10
F 9
F8
F 7
F6
F 5
F 4
F3
F2
F1
%0
5
3
* co
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning