A common way to make hydrogen gas in the laboratory is to place a metal such as zinc in hydrochloric acid (see the figure). The hydrochloric acid reacts with the metal to produce hydrogen gas, which is then collected over water. Suppose a student carries out this reaction and collects a total of 147.6 mL of gas at a pressure of 743 mmHg and a temperature of 25° C. What mass of hydrogen gas (in mg) does the student collect? (The vapor pressure of water is 23.78 mmHg at 25° C.) Collecting a Gas over Water -Н2 Hydrogen plus water vapor - Zn -HCl -Н-0 Express the mass to three significant figures and include the appropriate units. HA Value Units т 3
A common way to make hydrogen gas in the laboratory is to place a metal such as zinc in hydrochloric acid (see the figure). The hydrochloric acid reacts with the metal to produce hydrogen gas, which is then collected over water. Suppose a student carries out this reaction and collects a total of 147.6 mL of gas at a pressure of 743 mmHg and a temperature of 25° C. What mass of hydrogen gas (in mg) does the student collect? (The vapor pressure of water is 23.78 mmHg at 25° C.) Collecting a Gas over Water -Н2 Hydrogen plus water vapor - Zn -HCl -Н-0 Express the mass to three significant figures and include the appropriate units. HA Value Units т 3
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 152CP: A mixture of chromium and zinc weighing 0.362 g was reacted with an excess of hydrochloric acid....
Related questions
Question
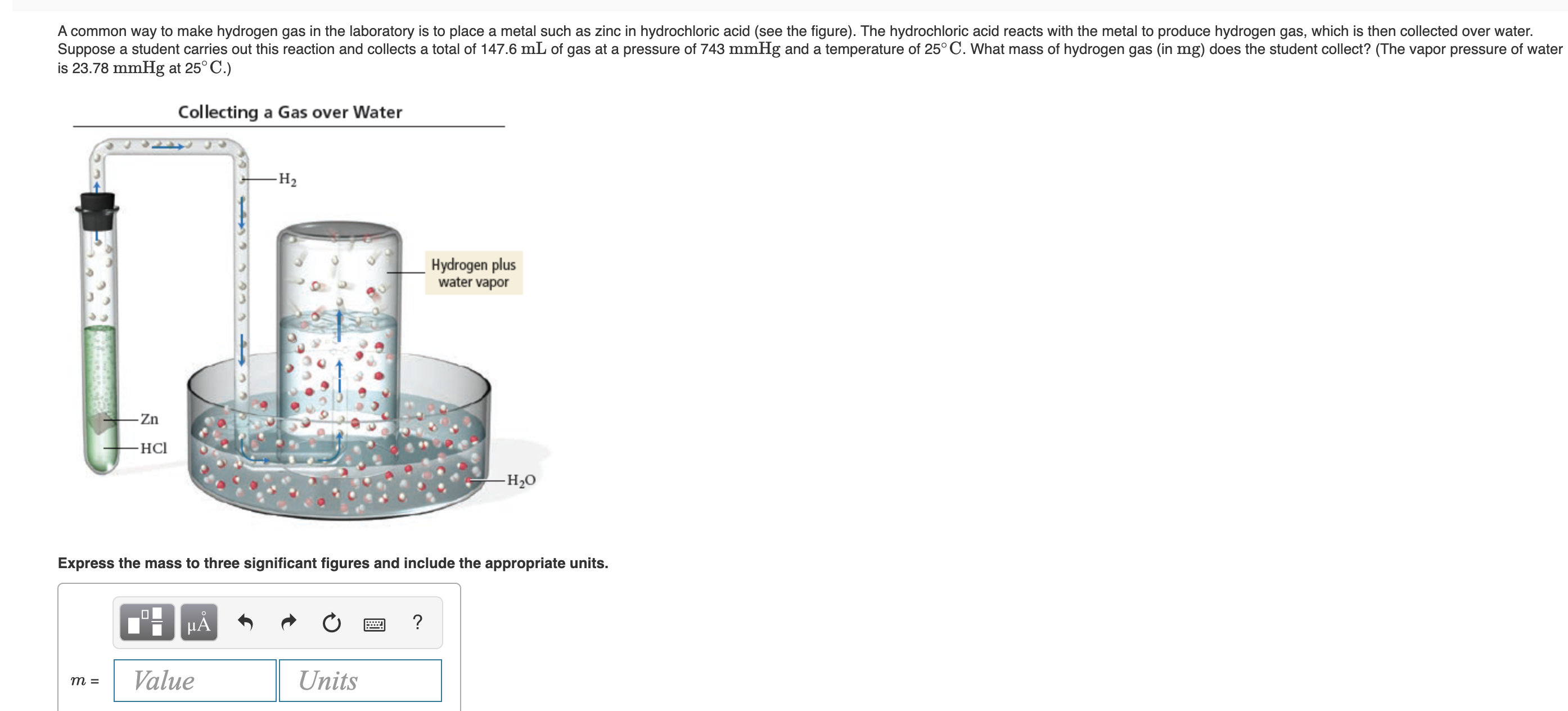
Transcribed Image Text:A common way to make hydrogen gas in the laboratory is to place a metal such as zinc in hydrochloric acid (see the figure). The hydrochloric acid reacts with the metal to produce hydrogen gas, which is then collected over water.
Suppose a student carries out this reaction and collects a total of 147.6 mL of gas at a pressure of 743 mmHg and a temperature of 25° C. What mass of hydrogen gas (in mg) does the student collect? (The vapor pressure of water
is 23.78 mmHg at 25° C.)
Collecting a Gas over Water
-Н2
Hydrogen plus
water vapor
- Zn
-HCl
-Н-0
Express the mass to three significant figures and include the appropriate units.
HA
Value
Units
т 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning